Question: The total energy eigenvalues for the hydrogen atom are given by E n = e 2 / (8Ïε 0 a 0 n), n = 1,
The total energy eigenvalues for the hydrogen atom are given by En= ˆ’e2 / (8πε0a0n), n = 1, 2, 3, 4,€¦, and the three quantum numbers associated with the total energy eigenfunctions are related by n = 1, 2, 3, 4,.....; l = 0,1 , 2, 3, ..., n ˆ’ 1; and ml = 0, ±1, ±2, ±3,... ± l, n = 1, 2, 3, 4,.....; l = 0,1 , 2, 3, ..., n ˆ’ 1; and ml = 0, ±1, ±2, ±3,€¦,±l. Using the nomenclature ,ψnlm1list all eigenfunctions that have the following total energy eigenvalues:
a.
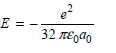
b.
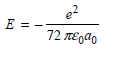
c.
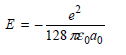
E = 32 TEgdo 72 TEgao
Step by Step Solution
3.34 Rating (169 Votes )
There are 3 Steps involved in it
a corresponds to n 2 The total energy eigenfunctions are 200 210 211 211 The level has a ... View full answer

Get step-by-step solutions from verified subject matter experts


