Question: The geometry compound has square planar geometry, whereas the CIF: compound has O XeFg, T-shaped O SF, trigonal planar OPCI, trigonal pyramidal O CCla, tetrahedral
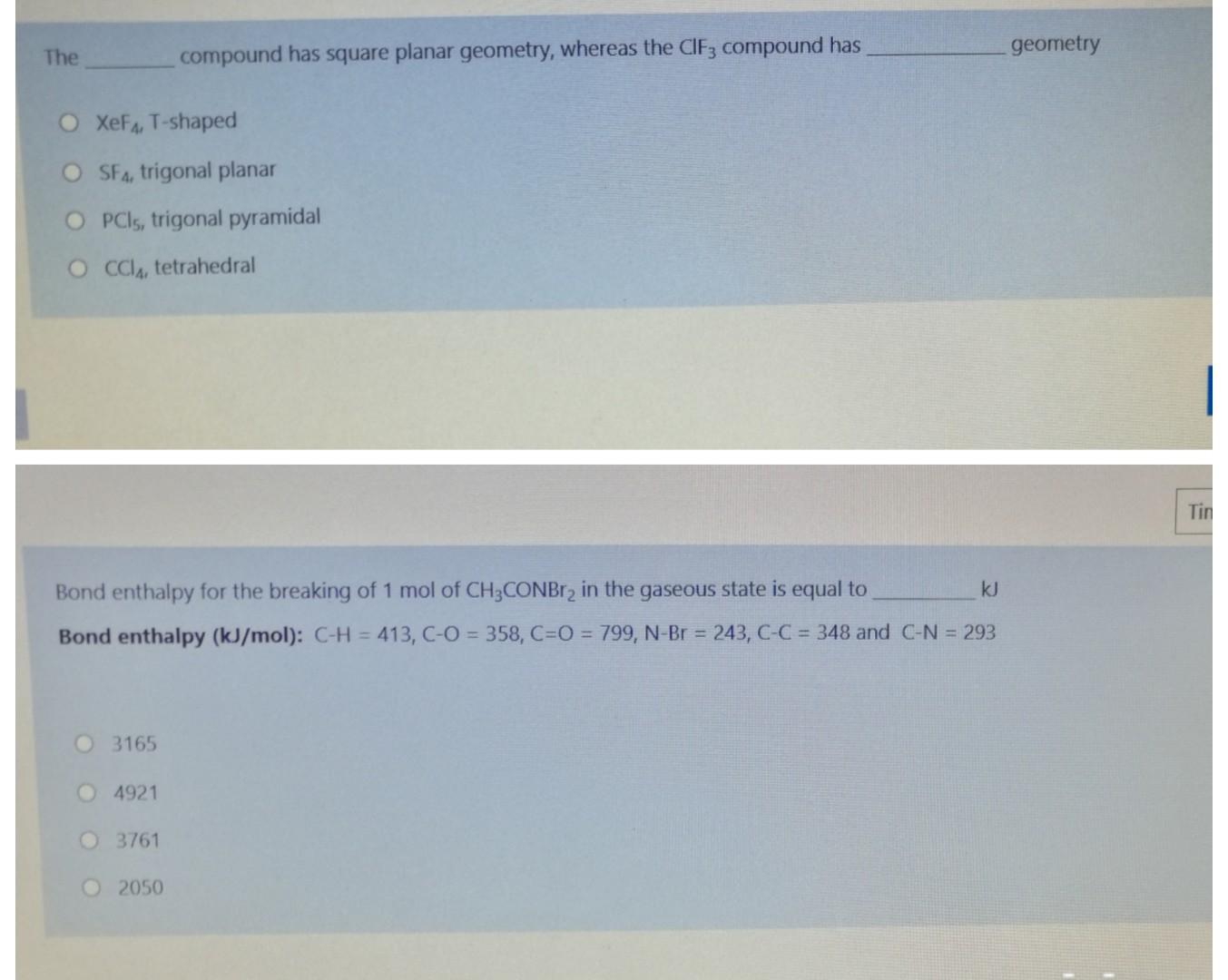
The geometry compound has square planar geometry, whereas the CIF: compound has O XeFg, T-shaped O SF, trigonal planar OPCI, trigonal pyramidal O CCla, tetrahedral Tin Bond enthalpy for the breaking of 1 mol of CH3CONBr2 in the gaseous state is equal to k] Bond enthalpy (kJ/mol): C-H = 413, C-O = 358, C=0 = 799, N-Br = 243, C-C = 348 and C-N = 293 O 3165 4921 3761 2050
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


