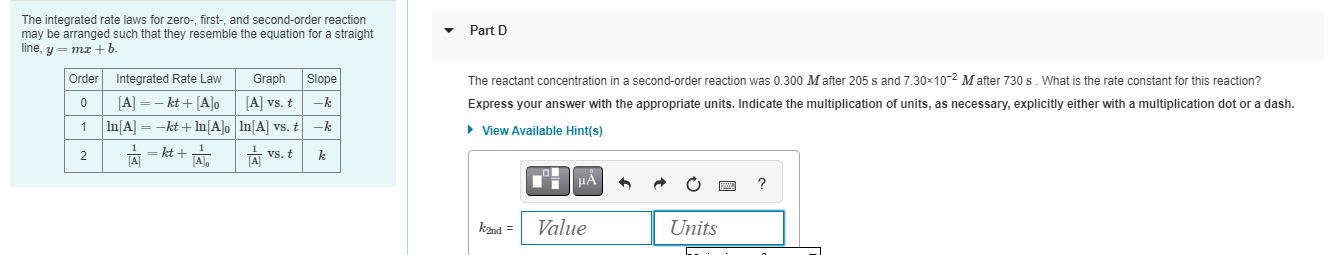
Question: The integrated rate laws for zero - , first - , and second - order reaction may be arranged such that they resemble the equation
The integrated rate laws for zero first and secondorder reaction may be arranged such that they resemble the equation for a straight line, ymxb
Order Integrated Rate Law Graph Slope
AktA
A vs t
k
lnAktlnA
lnA vs t
k
A ktA
A vs t
k
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


