Question: please help The integrated rate laws for zero-, first-, and secondorder reaction may be arranged such that they resemble the equation for a straight line,
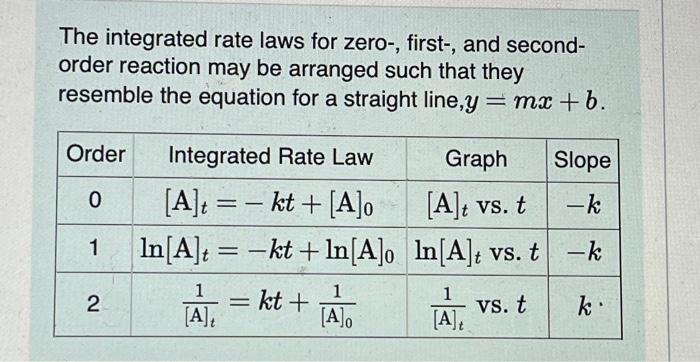
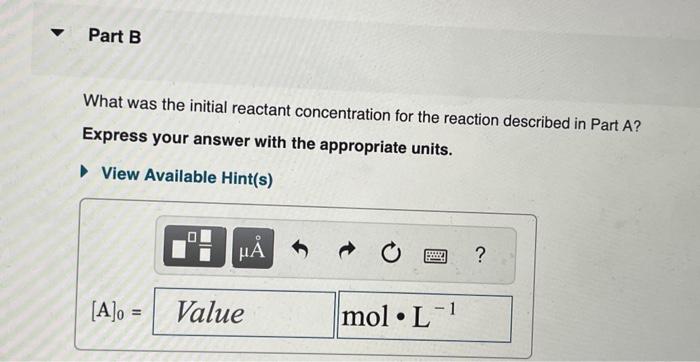

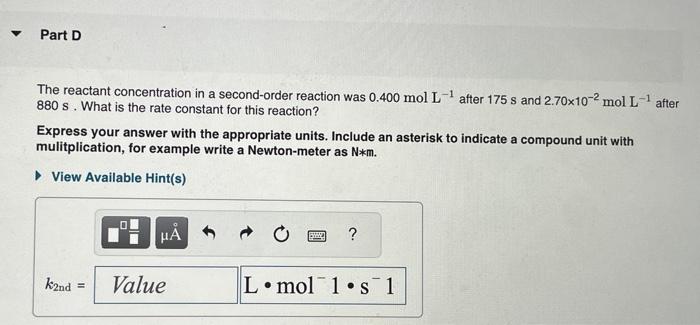
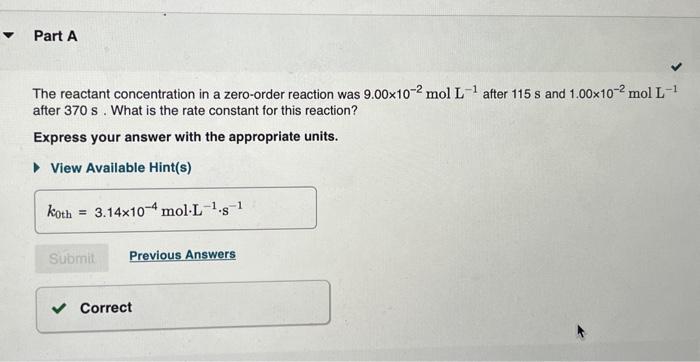
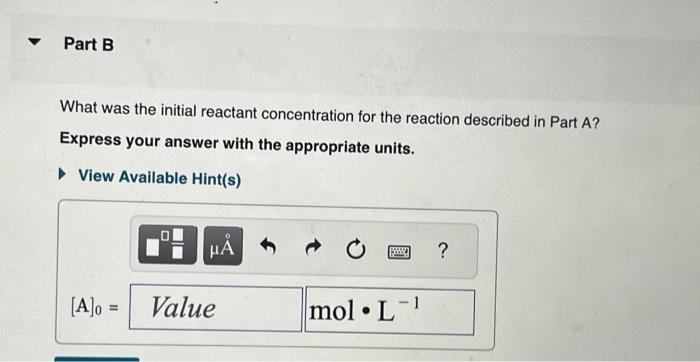
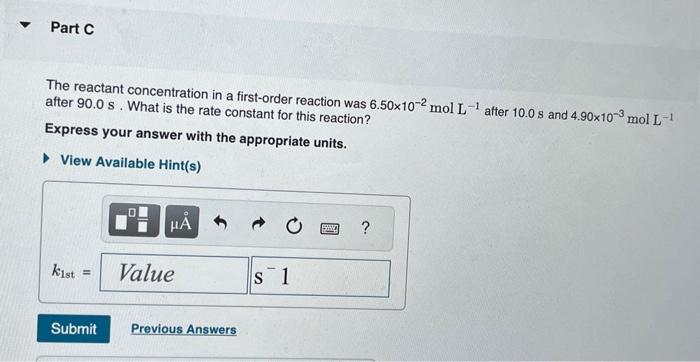
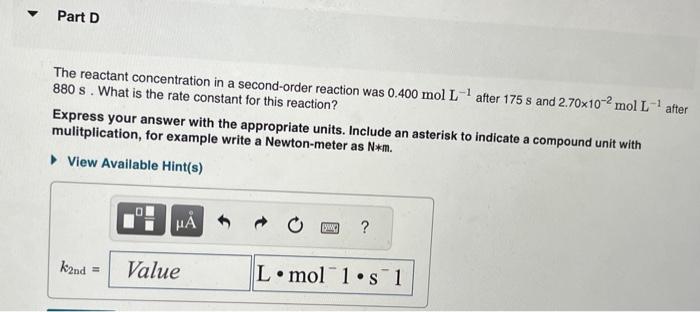
The integrated rate laws for zero-, first-, and secondorder reaction may be arranged such that they resemble the equation for a straight line, y=mx+b. What was the initial reactant concentration for the reaction described in Part A? Express your answer with the appropriate units. The reactant concentration in a first-order reaction was 6.50102molL1 after 10.0s and 4.90103molL1 after 90.0s. What is the rate constant for this reaction? Express your answer with the appropriate units. The reactant concentration in a second-order reaction was 0.400molL1 after 175s and 2.70102molL1 after 880s. What is the rate constant for this reaction? Express your answer with the appropriate units. Include an asterisk to indicate a compound unit with mulitplication, for example write a Newton-meter as Nm. The reactant concentration in a zero-order reaction was 9.00102molL1 after 115s and 1.00102molL1 after 370s. What is the rate constant for this reaction? Express your answer with the appropriate units. View Available Hint(s) k0th=3.14104molL1s1 What was the initial reactant concentration for the reaction described in Part A? Express your answer with the appropriate units. The reactant concentration in a first-order reaction was 6.50102molL1 after 10.0s and 4.90103molL1 after 90.0s. What is the rate constant for this reaction? Express your answer with the appropriate units. The reactant concentration in a second-order reaction was 0.400molL1 after 175s and 2.70102molL1 after 880s. What is the rate constant for this reaction? Express your answer with the appropriate units. Include an asterisk to indicate a compound unit with mulitplication, for example write a Newton-meter as Nm
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


