Question: The lattice enthalpy (formation of ionic solid from ions in the gas phase) for AgCl(s) is -916 kJ/mol and the hydration enthalpy (dissolution of
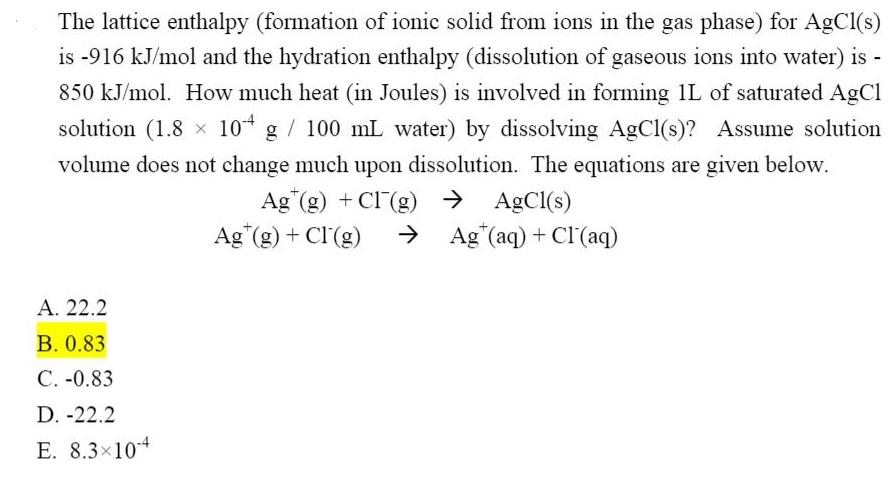
The lattice enthalpy (formation of ionic solid from ions in the gas phase) for AgCl(s) is -916 kJ/mol and the hydration enthalpy (dissolution of gaseous ions into water) is 850 kJ/mol. How much heat (in Joules) is involved in forming 1L of saturated AgCl solution (1.8 x 10* g / 100 mL water) by dissolving AgCl(s)? Assume solution volume does not change much upon dissolution. The equations are given below. Ag (g) + CI (g) AgCl(s) Ag (g) + Cl(g) > Ag"(aq) + Clr(aq) . 22.2 . 0.83 C. -0.83 D. -22.2 E. 8.3x104
Step by Step Solution
3.57 Rating (143 Votes )
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


