Question: The question calls for determination 3 on this table. thank you! 1. Rates of reactions are temperature dependent. When the temperature is decreased the time

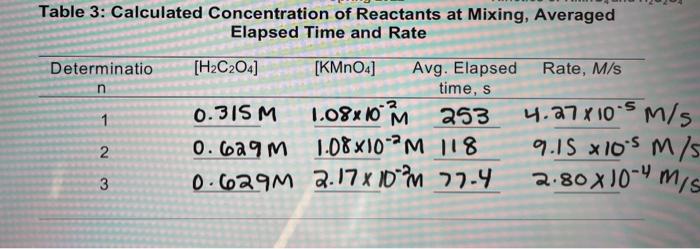
1. Rates of reactions are temperature dependent. When the temperature is decreased the time for the reaction to be completed is increased. For every 10 C decrease, the time increases two fold. a. Use Determination 3 of Table 3 and determine the new rate of the reaction. b. Use the rate of the reaction determined in the above question and now determine the rate constant at this new lower temperature. c. Is this new rate constant the same, higher, or lower than the experimentally determined rate constant? Explain. Table 3: Calculated Concentration of Reactants at Mixing, Averaged Elapsed Time and Rate Determinatio n 1 [H2C204] [KMnO4] Avg. Elapsed Rate, M/s time, s 0.315M 1.08x10 m 253 4.27x10-5 m/s 0.629 m 1.08x10-2M 118 9.15 x 105 m/s 0.629M 2.17% 10% 77-4 2 3 2.80X10mis 1. Rates of reactions are temperature dependent. When the temperature is decreased the time for the reaction to be completed is increased. For every 10 C decrease, the time increases two fold. a. Use Determination 3 of Table 3 and determine the new rate of the reaction. b. Use the rate of the reaction determined in the above question and now determine the rate constant at this new lower temperature. c. Is this new rate constant the same, higher, or lower than the experimentally determined rate constant? Explain. Table 3: Calculated Concentration of Reactants at Mixing, Averaged Elapsed Time and Rate Determinatio n 1 [H2C204] [KMnO4] Avg. Elapsed Rate, M/s time, s 0.315M 1.08x10 m 253 4.27x10-5 m/s 0.629 m 1.08x10-2M 118 9.15 x 105 m/s 0.629M 2.17% 10% 77-4 2 3 2.80X10mis
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


