Question: The reactant A decomposes to form an intermediate B, which further decomposes to form a final product C in the following reaction process: A
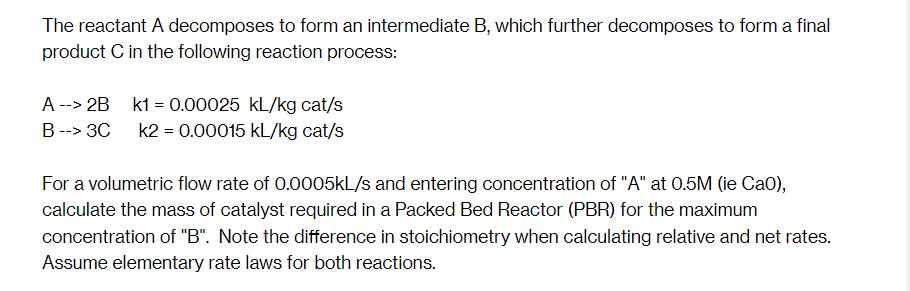
The reactant A decomposes to form an intermediate B, which further decomposes to form a final product C in the following reaction process: A --> 2B k1 = 0.00025 kl/kg cat/s k2 = 0.00015 kl/kg cat/s B --> 3C For a volumetric flow rate of 0.0005kL/s and entering concentration of "A" at 0.5M (ie Cao), calculate the mass of catalyst required in a Packed Bed Reactor (PBR) for the maximum concentration of "B". Note the difference in stoichiometry when calculating relative and net rates. Assume elementary rate laws for both reactions.
Step by Step Solution
3.39 Rating (155 Votes )
There are 3 Steps involved in it
SOLUTION To start lets break down the reaction process A decomposes to form B A 2B rate equation k1A B further decomposes to form C B 3C rate equation k2B We know that the volumetric flow rate is 0000... View full answer

Get step-by-step solutions from verified subject matter experts


