Question: The reactant concentration in a zero-order reaction was 0.100 mol L 1 after 190 s and 1.5010 2 mol L 1 after 355 s .
 The reactant concentration in a zero-order reaction was 0.100 mol L−1 after 190 s and 1.50×10−2 mol L−1 after 355 s . What is the rate constant for this reaction?
The reactant concentration in a zero-order reaction was 0.100 mol L−1 after 190 s and 1.50×10−2 mol L−1 after 355 s . What is the rate constant for this reaction?
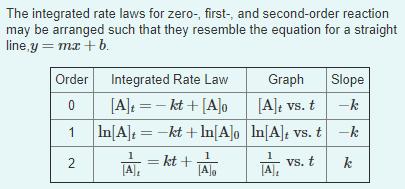
The integrated rate laws for zero-, first-, and second-order reaction may be arranged such that they resemble the equation for a straight line,y = mx + b. Order Integrated Rate Law Graph Slope [A]: =- kt + [A]o [A]t vs. t -k In[A] =-kt + In[A]o In[A]t vs. t-k TA, = kt TA, vs. t k 2.
Step by Step Solution
3.40 Rating (150 Votes )
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts
Document Format (2 attachments)
635e352ed0d53_182362.pdf
180 KBs PDF File
635e352ed0d53_182362.docx
120 KBs Word File


