Question: The reversible elementary liquid phase reaction A + B takes place isobarically and isothermally in a PBR over a catalyst. At the operating temperature, the
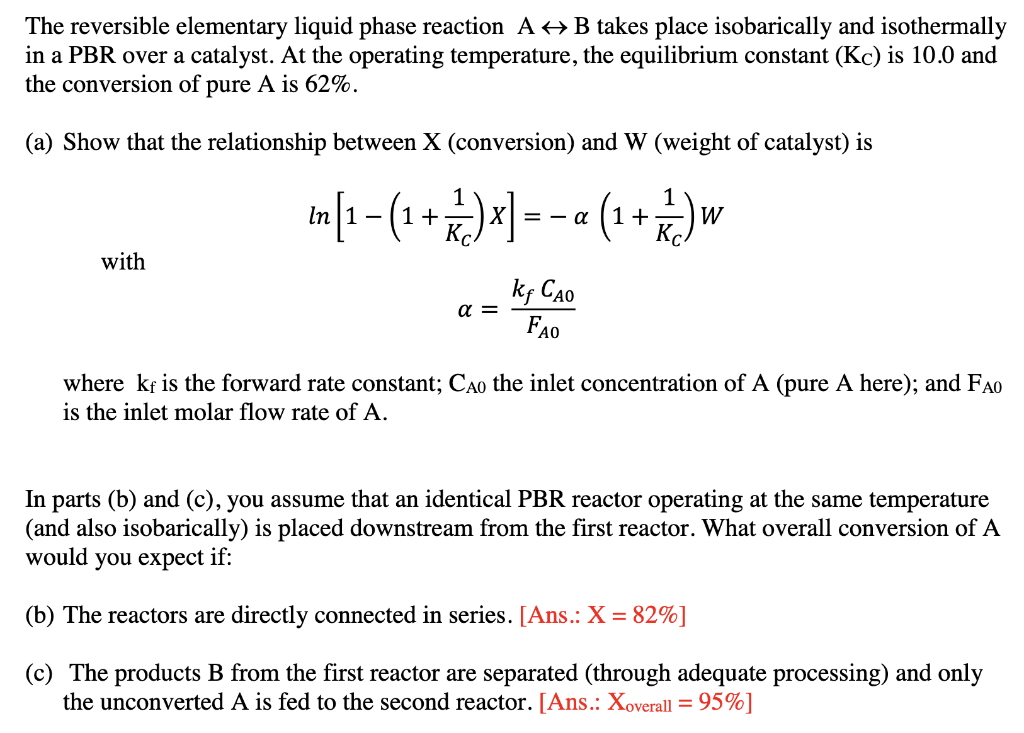
The reversible elementary liquid phase reaction A + B takes place isobarically and isothermally in a PBR over a catalyst. At the operating temperature, the equilibrium constant (Kc) is 10.0 and the conversion of pure A is 62%. (a) Show that the relationship between X (conversion) and W (weight of catalyst) is 1 In [1-(1+++)x] =-a (1+r) w W with kg Cao a = FAO where kf is the forward rate constant; Cao the inlet concentration of A (pure A here); and FAO is the inlet molar flow rate of A. In parts (b) and (c), you assume that an identical PBR reactor operating at the same temperature (and also isobarically) is placed downstream from the first reactor. What overall conversion of A would you expect if: (b) The reactors are directly connected in series. [Ans.: X = 82%] (c) The products B from the first reactor are separated (through adequate processing) and only the unconverted A is fed to the second reactor. [Ans.: Xoverall = 95%]
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


