Question: The reversible first order mas ptiase reaction, 4 , 7 is to be carried out in a constant volume batich reactor at 300K. Find the
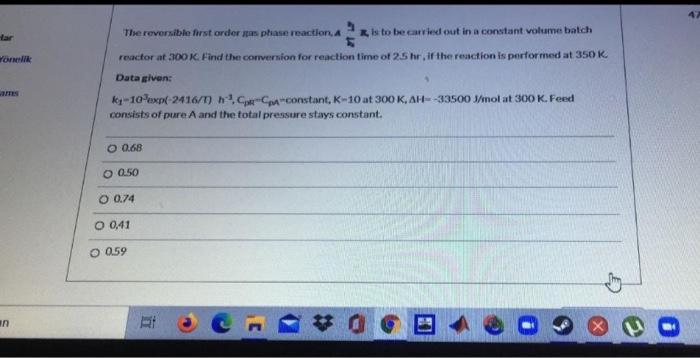
The reversible first order mas ptiase reaction, 4 , 7 is to be carried out in a constant volume batich reactor at 300K. Find the corversion for reaction time of 25hr, if the reaction is performed at 350K. Data given: k1103exp(2416/T)h3,CpKCm= eonstant, K10 at 300K,H=33500J/mol at 300K. Feed consists of pure A and the total pressure stays constant. 0.68 0.50 0.74 0,41 0.59
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


