Question: There are 3 unknown solution in the chemistry lab. Some supplies you have on hand are a conductivity probe, a pH meter, various indicators and
There are 3 unknown solution in the chemistry lab. Some supplies you have on hand are a conductivity probe, a pH meter, various indicators and a solution of .1M NaOH.
a. Design a plan in order to identify the solutions and be able to distinguish the differences between them.
b. What tests would you conduct in the lab? What exactly would you do and what data would you collect?
c. Is it even possible to identify all the solutions? Why or why not?
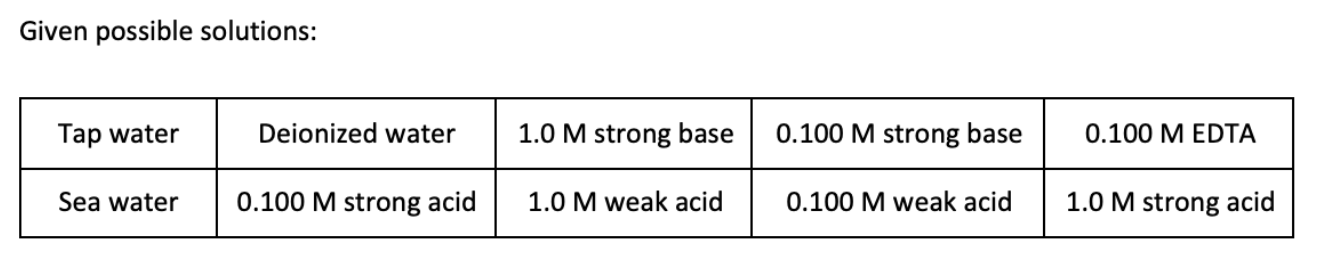
Given possible solutions: Tap water Deionized water 1.0 M strong base 0.100 M strong base 0.100 M EDTA Sea water 0.100 M strong acid 1.0 M weak acid 0.100 M weak acid 1.0 M strong acid
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


