Question: There are four common types of solids: ionic, network covalent, metallic, and molecular. ( a ) For each of these types of solids, identify the
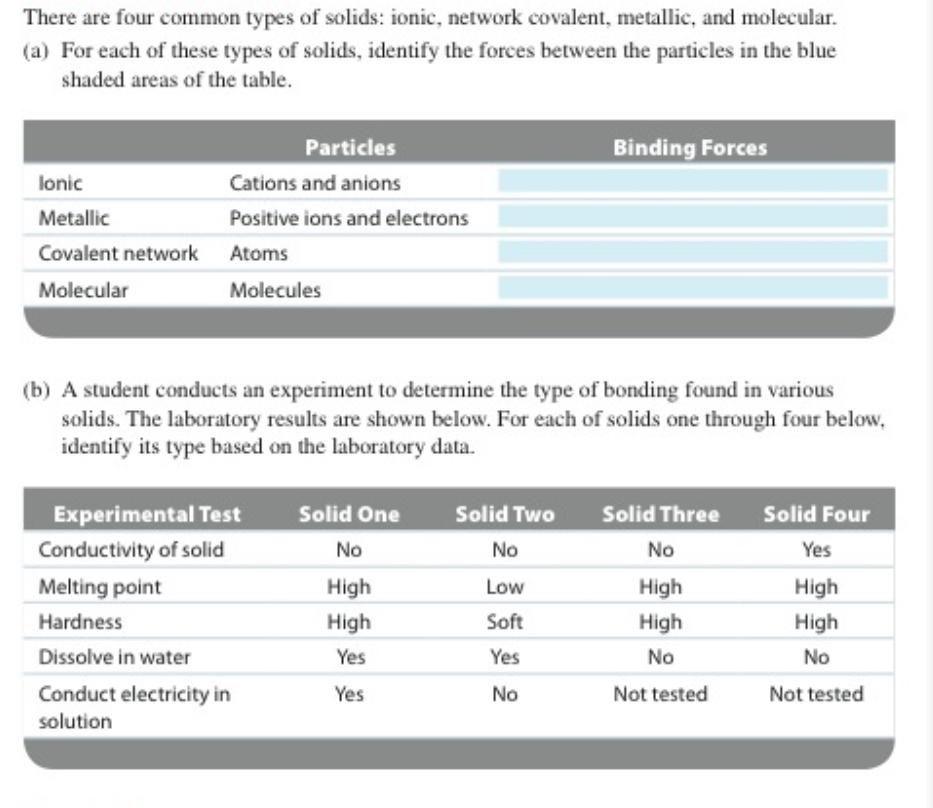
There are four common types of solids: ionic, network covalent, metallic, and molecular.
a For each of these types of solids, identify the forces between the particles in the blue shaded areas of the table.
tableParticles,Binding ForcesIonicCations and anions,MetallicPositive ions and electrons,Covalent network,Atoms,MolecularMolecules,
b A student conducts an experiment to determine the type of bonding found in various solids. The laboratory results are shown below. For each of solids one through four below, identify its type based on the laboratory data.
tableExperimental Test,Solid One,Solid Two,Solid Three,Solid FourConductivity of solid,NoNoNoYesMelting point,High,Low,High,HighHardnessHigh,Soft,High,HightableDissolve in waterYes,Yes,NoNotableConduct electricity insolutionYes,NoNot tested,Not tested
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


