Question: this is a chemcad homework. please take the selecting data in tables. thank you.. Methanol is manufactured in a synthesis loop, in which a mixture
this is a chemcad homework. please take the selecting data in tables. thank you..
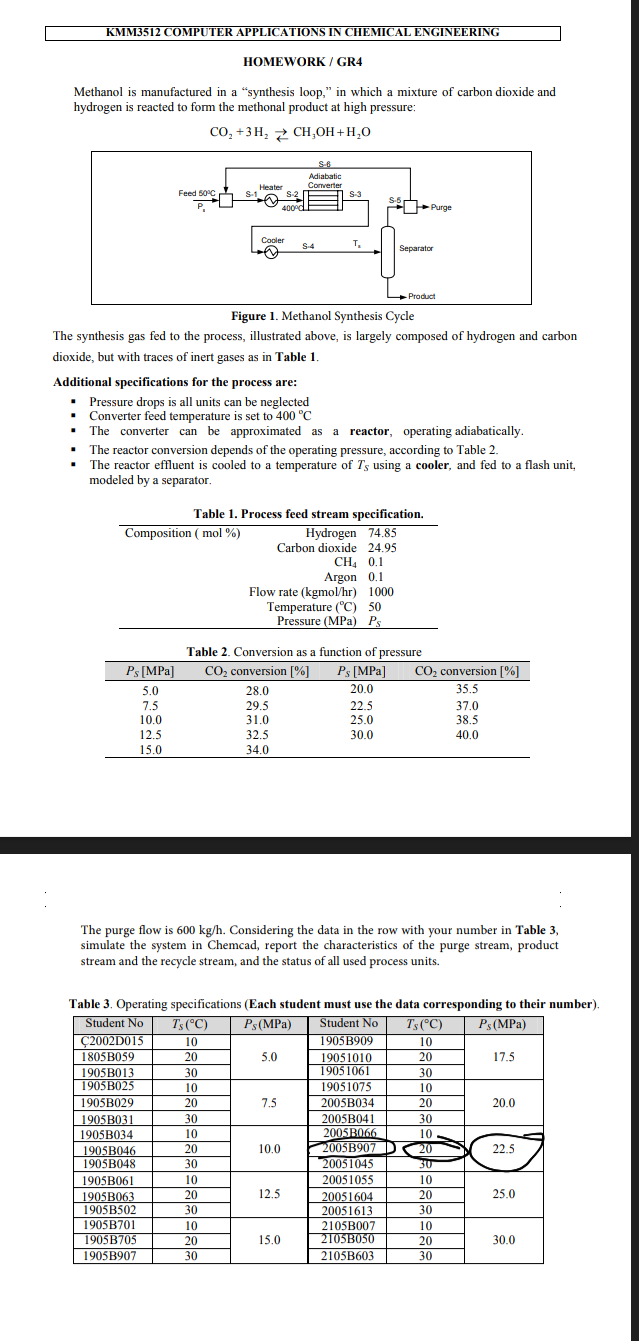
Methanol is manufactured in a "synthesis loop," in which a mixture of carbon dioxide and hydrogen is reacted to form the methonal product at high pressure: CO2+3H2CH3OH+H2O Figure 1. Methanol Synthesis Cycle The synthesis gas fed to the process, illustrated above, is largely composed of hydrogen and carbon dioxide, but with traces of inert gases as in Table 1. Additional specifications for the process are: - Pressure drops is all units can be neglected - Converter feed temperature is set to 400C - The converter can be approximated as a reactor, operating adiabatically. - The reactor conversion depends of the operating pressure, according to Table 2. - The reactor effluent is cooled to a temperature of TS using a cooler, and fed to a flash unit, modeled by a separator. Table 2. Conversion as a function of pressure The purge flow is 600kg/h. Considering the data in the row with your number in Table 3 , simulate the system in Chemcad, report the characteristics of the purge stream, product stream and the recycle stream, and the status of all used process units. Table 3. Operating specifications (Each student must use the data corresponding to their number)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


