Question: This is a Reaction/Chemical Reactor Engineering question. Please provide full, clear working for the answer with legible handwriting and explanation, if needed. Please know I
This is a Reaction/Chemical Reactor Engineering question. Please provide full, clear working for the answer with legible handwriting and explanation, if needed.
Please know I am depending on Chegg to learn and pass this course. Thank you so much.
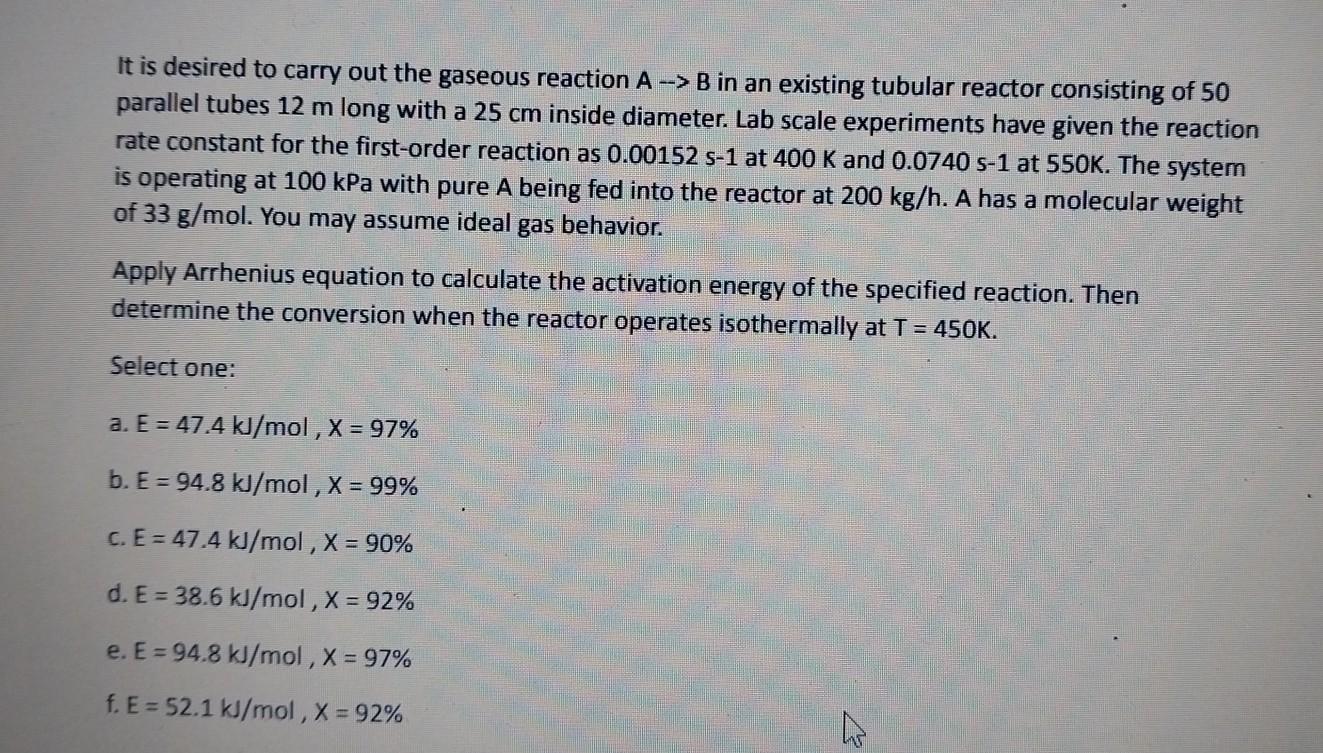
It is desired to carry out the gaseous reaction AB in an existing tubular reactor consisting of 50 parallel tubes 12m long with a 25cm inside diameter. Lab scale experiments have given the reaction rate constant for the first-order reaction as 0.00152s1 at 400K and 0.0740s1 at 550K. The system is operating at 100kPa with pure A being fed into the reactor at 200kg/h. A has a molecular weight of 33g/mol. You may assume ideal gas behavior. Apply Arrhenius equation to calculate the activation energy of the specified reaction. Then determine the conversion when the reactor operates isothermally at T=450K. Select one: a. E=47.4kJ/mol,X=97% b. E=94.8kJ/mol,X=99% c. E=47.4kJ/mol,X=90% d. E=38.6kJ/mol,X=92% e. E=94.8kJ/mol,X=97% f.E=52.1kJ/mol,X=92%
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


