Question: Try it here, using the strategy below Use the curved arrow formalism to show how the electrons flow in the resonance form on the left
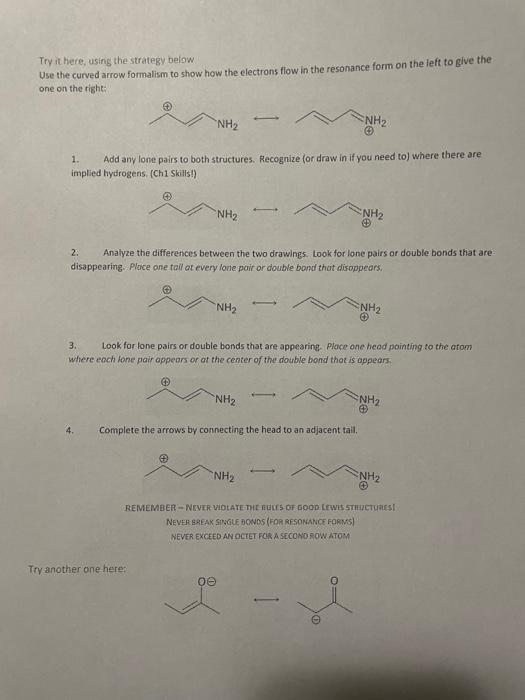
Try it here, using the strategy below Use the curved arrow formalism to show how the electrons flow in the resonance form on the left to give the one on the right: "NH2 NH2 1. Add any lone pairs to both structures. Recognize (or draw in if you need to) where there are implied hydrogens (Chi Skills!) NH2 NH2 2. Analyze the differences between the two drawings. Look for lone pairs or double bonds that are disappearing. Place one tall at every lone pair or double bond that disappears. NH2 NH2 3. Look for lone pairs or double bonds that are appearing place one head pointing to the atom where each lone pair appears or at the center of the double bond that is appears. NH2 NH2 4. Complete the arrows by connecting the head to an adjacent tail. "NH2 REMEMBER-NEVER VIOLATE THE DES OF GOOD LOWESTICTURES NEVER BREAK SINGLE BONDS (FOR RESONANCE FORVS) NEVER EXCEED AN OCTET FOR A SECOND ROW ATOM Try another one here: ve
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


