Question: Two liquid phase reactions occur in a CSTR operating at steady state: A+BCA+1/2CDrA1=k1CAArA2=k2CACC1/2HRx1A=20kcal/molAHR2A=5kcal/molA The feed conditions are CA0=CB=4M,T0=50C, and =4min. The heat capacity and density
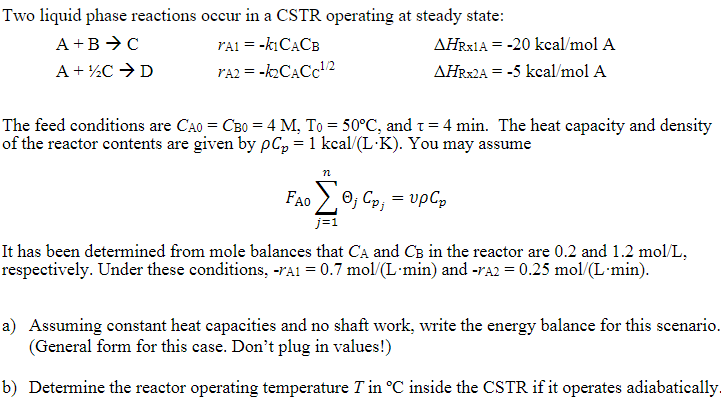
Two liquid phase reactions occur in a CSTR operating at steady state: A+BCA+1/2CDrA1=k1CAArA2=k2CACC1/2HRx1A=20kcal/molAHR2A=5kcal/molA The feed conditions are CA0=CB=4M,T0=50C, and =4min. The heat capacity and density of the reactor contents are given by Cp=1kcal/(LK). You may assume FA0j=1njCpj=vCp It has been determined from mole balances that CA and CB in the reactor are 0.2 and 1.2mol/L, respectively. Under these conditions, rA1=0.7mol/(Lmin) and rA2=0.25mol/(Lmin). a) Assuming constant heat capacities and no shaft work, write the energy balance for this scenario. (General form for this case. Don't plug in values!) b) Determine the reactor operating temperature T in C inside the CSTR if it operates adiabatically. Two liquid phase reactions occur in a CSTR operating at steady state: A+BCA+1/2CDrA1=k1CAArA2=k2CACC1/2HRx1A=20kcal/molAHR2A=5kcal/molA The feed conditions are CA0=CB=4M,T0=50C, and =4min. The heat capacity and density of the reactor contents are given by Cp=1kcal/(LK). You may assume FA0j=1njCpj=vCp It has been determined from mole balances that CA and CB in the reactor are 0.2 and 1.2mol/L, respectively. Under these conditions, rA1=0.7mol/(Lmin) and rA2=0.25mol/(Lmin). a) Assuming constant heat capacities and no shaft work, write the energy balance for this scenario. (General form for this case. Don't plug in values!) b) Determine the reactor operating temperature T in C inside the CSTR if it operates adiabatically
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


