Question: Use 5 kJ/mol for enthalpy value rather than the value given. 6.11 Pure ethanol has a melting temperature of Tm = 159 K at p
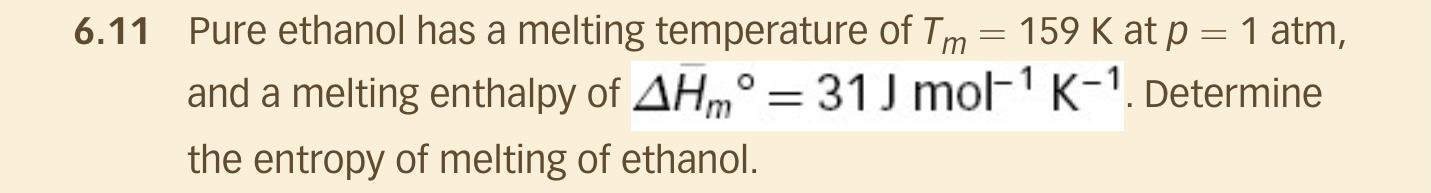 Use 5 kJ/mol for enthalpy value rather than the value given.
Use 5 kJ/mol for enthalpy value rather than the value given.
6.11 Pure ethanol has a melting temperature of Tm = 159 K at p = 1 atm, and a melting enthalpy of AHm = 31 J mol-? K-1. Determine the entropy of melting of ethanol. =
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


