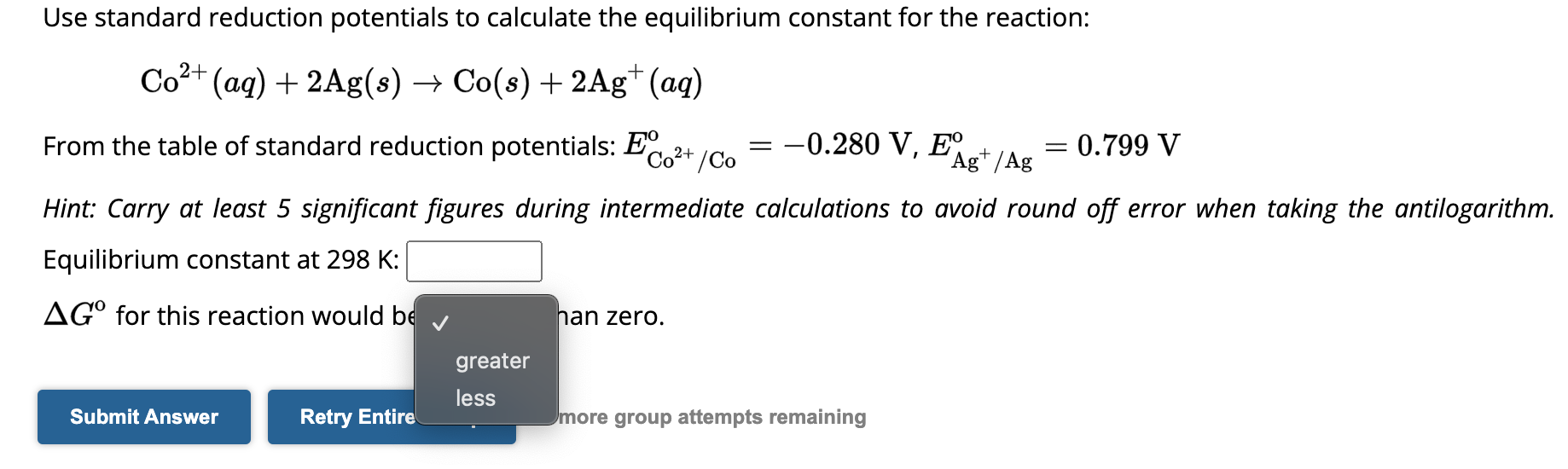
Question: Use standard reduction potentials to calculate the equilibrium constant for the reaction: C o 2 + ( a q ) + 2 A g (
Use standard reduction potentials to calculate the equilibrium constant for the reaction:
From the table of standard reduction potentials:
Hint: Carry at least significant figures during intermediate calculations to avoid round off error when taking the antilogarithm.
Equilibrium constant at :
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


