Question: Use standard reduction potentials to find the equilibrium constant for the following reaction at 25C : Mg2+(aq)+Pb(s)Mg(s)+Pb2+(aq) Enter your answer in scientific notation to one
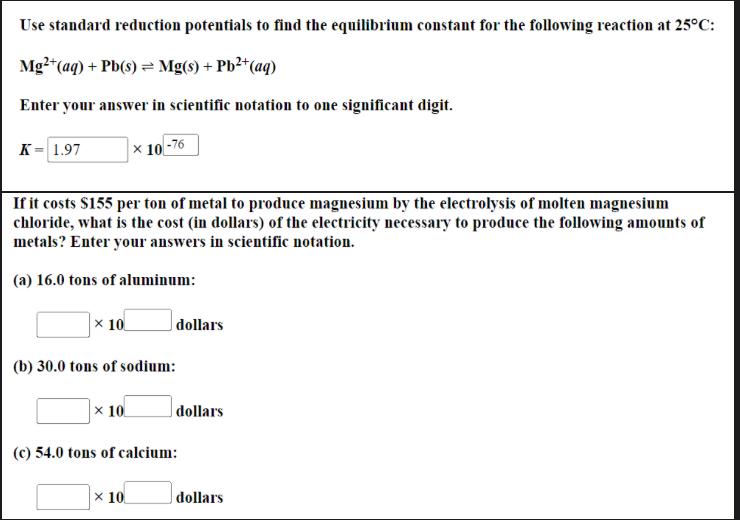
Use standard reduction potentials to find the equilibrium constant for the following reaction at 25C : Mg2+(aq)+Pb(s)Mg(s)+Pb2+(aq) Enter your answer in scientific notation to one significant digit. K=10 If it costs S155 per ton of metal to produce magnesium by the electrolysis of molten magnesium chloride, what is the cost (in dollars) of the electricity necessary to produce the following amounts of metals? Enter your answers in scientific notation. (a) 16.0 tons of aluminum: 10dollars (b) 30.0 tons of sodium: 10dollars (c) 54.0 tons of calcium: 10dollars Use standard reduction potentials to find the equilibrium constant for the following reaction at 25C : Mg2+(aq)+Pb(s)Mg(s)+Pb2+(aq) Enter your answer in scientific notation to one significant digit. K=10 If it costs S155 per ton of metal to produce magnesium by the electrolysis of molten magnesium chloride, what is the cost (in dollars) of the electricity necessary to produce the following amounts of metals? Enter your answers in scientific notation. (a) 16.0 tons of aluminum: 10dollars (b) 30.0 tons of sodium: 10dollars (c) 54.0 tons of calcium: 10dollars
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


