Question: Use the following data to answer the items below: mass of unknown nonelectrolyte = 1 614 g freezing point of pure solvent = 0.05C mass
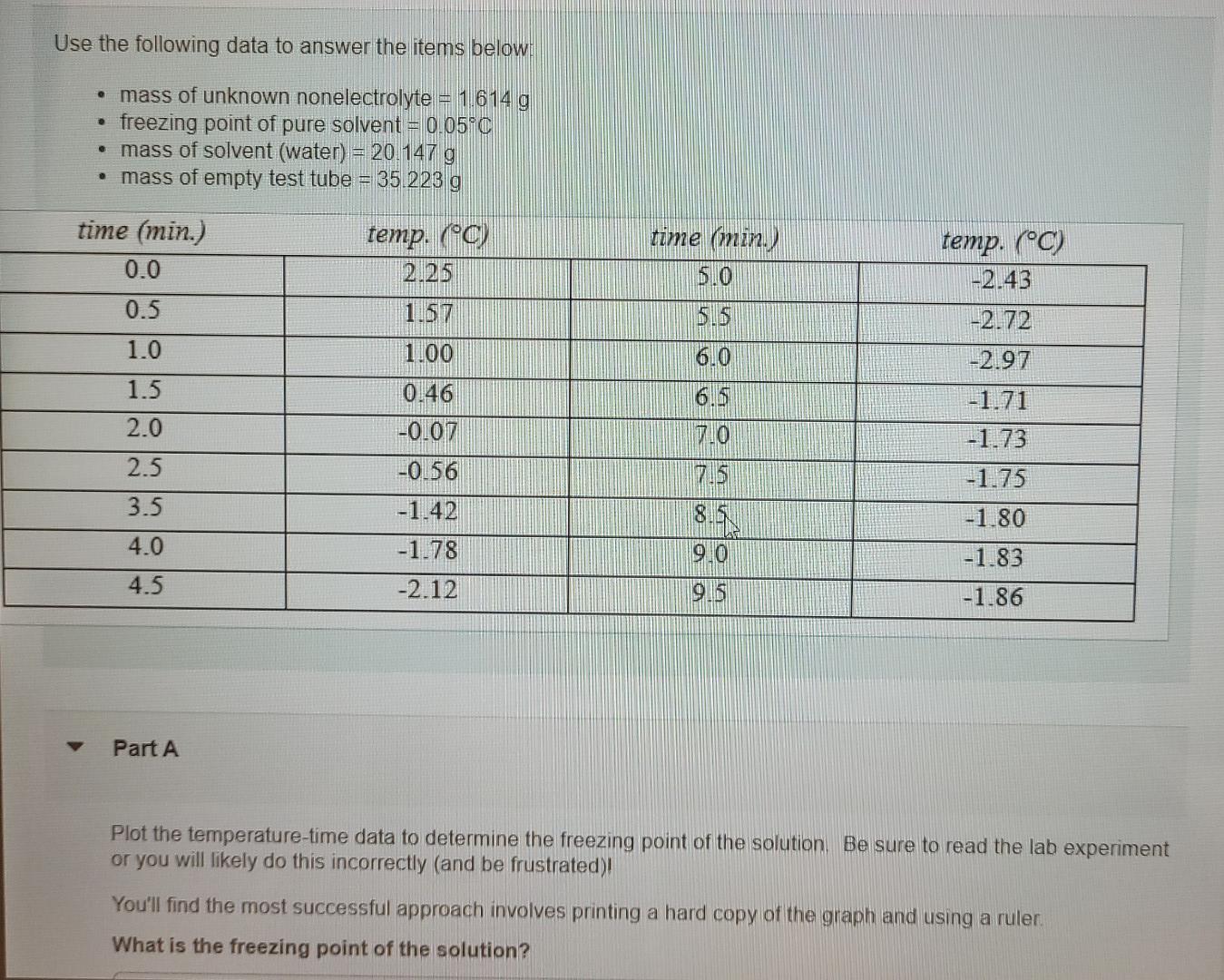
Use the following data to answer the items below: mass of unknown nonelectrolyte = 1 614 g freezing point of pure solvent = 0.05C mass of solvent (water) = 20.147 g mass of empty test tube = 35.223 g time (min.) 8.0 5.5 6.0 time (min.) 0.0 0.5 1.0 1.5 2.0 2.5 3.5 4.0 temp. (0) 2.25 1.57 1.00 0.46 -0.07 -0.56 -1.42 -1.78 -2.12 temp. (C) -2.43 -2.72 -2.97 -1.71 -1.73 -1.75 -1.80 7.0 TIB Ou 9.0 -1.83 4.5 OS -1.86 Part A Plot the temperature-time data to determine the freezing point of the solution. Be sure to read the lab experiment or you will likely do this incorrectly (and be frustrated)! You'll find the most successful approach involves printing a hard copy of the graph and using a ruler. What is the freezing point of the solution
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


