Question: Use the plot to evaluate the second order rate constant for the reaction of paratrifluoromethylbenzoic acid with diphenyldiazomethane (for para- CF3,x= 0.54) and its rate
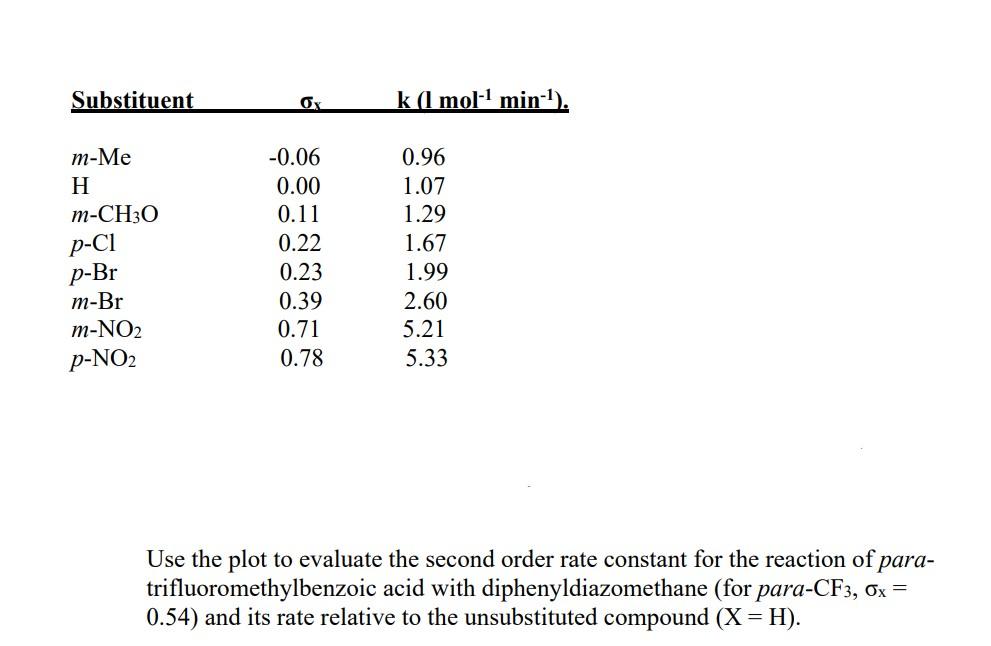
Use the plot to evaluate the second order rate constant for the reaction of paratrifluoromethylbenzoic acid with diphenyldiazomethane (for para- CF3,x= 0.54) and its rate relative to the unsubstituted compound (X=H)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


