Question: Use the References to access important values i net 96 A 0.5240 g sample of a pure soluble chloride compound is dissolved in water, and
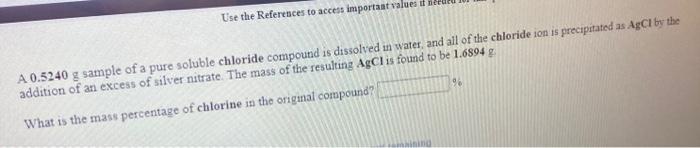
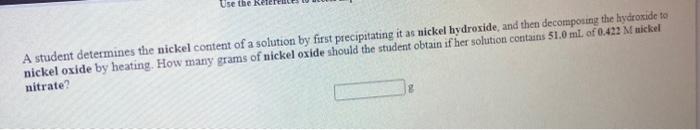
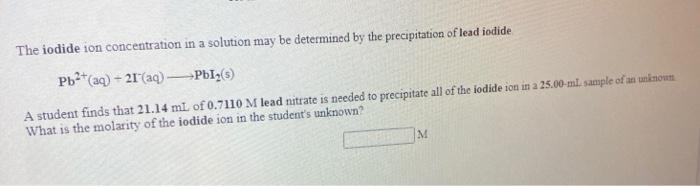
Use the References to access important values i net 96 A 0.5240 g sample of a pure soluble chloride compound is dissolved in water, and all of the chloride ion is precipitated as AgCl by the addition of an excess of silver nitrate. The mass of the resulting AgCl is found to be 1.6894 g. What is the mass percentage of chlorine in the original compound? Use the Re A student determines the nickel content of a solution by first precipitating it as nickel hydroxide, and then decomposing the hydroxide to nickel oxide by heating. How many grams of nickel oxide should the student obtain if her solution contains 51.0 ml. of 0.422 M wickel nitrate? The iodide ion concentration in a solution may be determined by the precipitation of lead iodide Pb2+ (aq) 21 (aq) Pb12(5) A student finds that 21.14 ml of 0.7110 M lead nitrate is needed to precipitate all of the lodide ion in a 25.00-ml. sample of an unknown What is the molarity of the iodide ion in the student's unknown? M
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


