Question: Use the References to access important values if needed for this question. According to the ideal gas law, a 1.057 mol sample of carbon dioxide
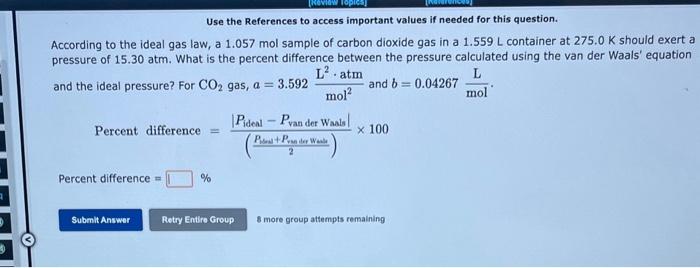
Use the References to access important values if needed for this question. According to the ideal gas law, a 1.057 mol sample of carbon dioxide gas in a 1.559L container at 275.0K should exert a pressure of 15.30atm. What is the percent difference between the pressure calculated using the van der Waals' equation and the ideal pressure? For CO2 gas, a=3.592mol2L2atm and b=0.04267molL. Percentdifference=(2Psoss+PramderWot)PidealPvanderWaals100 Percent difference = 8 more group attempts remaining
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


