Question: Use the References to access important values if needed for this question. The gas phase decomposition of hydrogen peroxide at 400C H2O2(g)H2O(g)+1/2O2(g) is second order
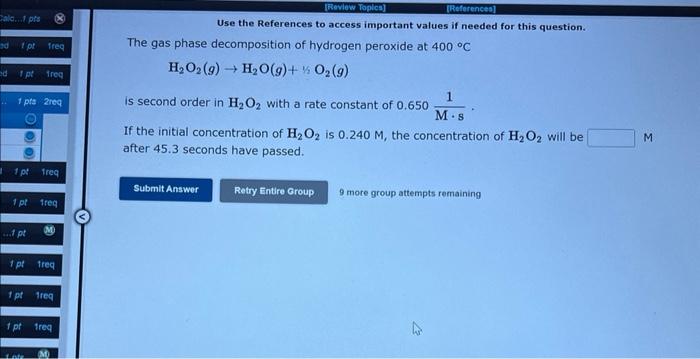
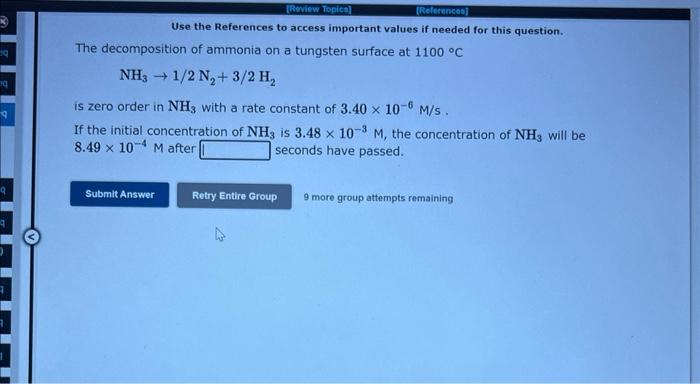
Use the References to access important values if needed for this question. The gas phase decomposition of hydrogen peroxide at 400C H2O2(g)H2O(g)+1/2O2(g) is second order in H2O2 with a rate constant of 0.650Ms1. If the initial concentration of H2O2 is 0.240M, the concentration of H2O2 will be after 45.3 seconds have passed. 9 mote group aftempts remaining The decomposition of ammonia on a tungsten surface at 1100C NH31/2N2+3/2H2 is zero order in NH3 with a rate constant of 3.40106M/s. If the initial concentration of NH3 is 3.48103M, the concentration of NH3 will be 8.49104M after seconds have passed
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


