Question: Use the References to access important values if needed for this question. In the laboratory you dissolve 12.0g of zinc iodide in a volumetric flask
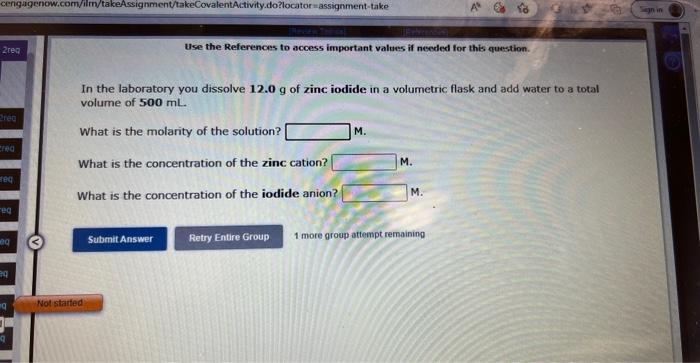
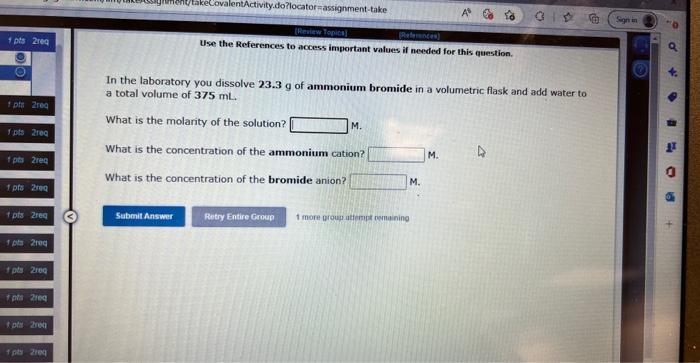
Use the References to access important values if needed for this question. In the laboratory you dissolve 12.0g of zinc iodide in a volumetric flask and add water to a total volume of 500mL What is the molarity of the solution? M. What is the concentration of the zinc cation? M. What is the concentration of the iodide anion? M. Use the References to access important values if needed for this question. In the laboratory you dissolve 23.3g of ammonium bromide in a volumetric flask and add water to a total volume of 375mL. What is the molarity of the solution? M. What is the concentration of the ammonium cation? M. What is the concentration of the bromide anion? M
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


