Question: Use the References to access important values if needed for this question. A student proposed the following mechanism for the gas phase reaction of fluorine
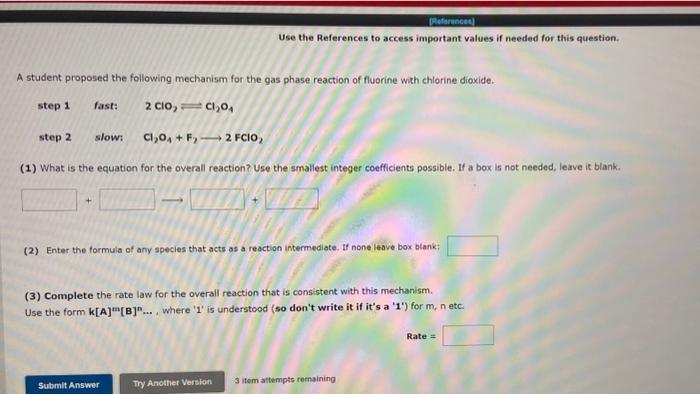
Use the References to access important values if needed for this question. A student proposed the following mechanism for the gas phase reaction of fluorine with chlorine dioxide. step 1 fast: 2ClO2Cl2O4 step2 slowi Cl2O4+F22FClO2 (1) What is the equation for the overall reaction? Use the smallest integer coefficients possible. If a box is not needed, leave it blank. (2) Enter the formula of any species that acts as a reaction intermediate. If none leave box blank: (3) Complete the rate law for the overall reaction that is consistent with this mechanism. Use the form k[A]m[B]n, where ' 1 ' is understood (so don't write it if it's a ' 1 ) for m, n etc. Rate = 3 item attempts remaining
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


