Question: Use the References to access important values if needed for this question. When silicon tetrafuoride reacts with water, hydrofluoric acid and silicon dioxide are produced.
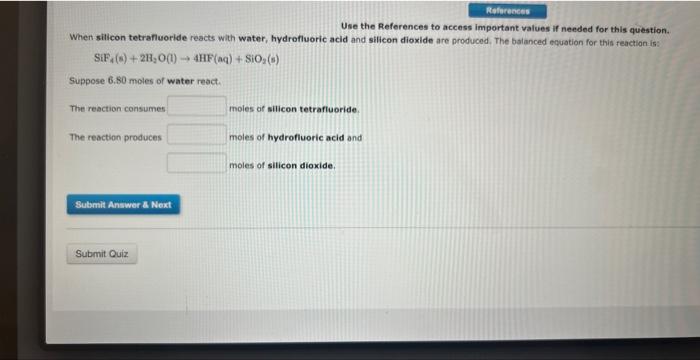
Use the References to access important values if needed for this question. When silicon tetrafuoride reacts with water, hydrofluoric acid and silicon dioxide are produced. The balanced equotion for this reaction is: SiF4(s)+2H2O(l)4HF(aq)+SiO2(s) Suppose 6, 80 moles of water react. The reaction consumes moles of silicon tetrafluoride. The reaction produces moles of hydrofluoric acid and moles of silicon diloxide
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


