Question: Use the References to access important values if needed for this question. The gas phase decomposition of hydrogen peroxide at 400C H2O2(g)H2O(g)+12O2(g) is second order
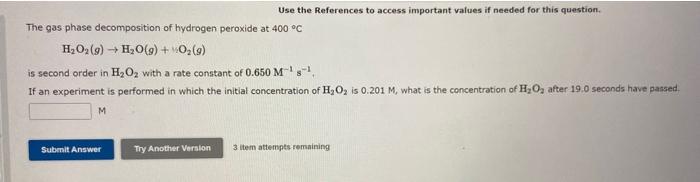
Use the References to access important values if needed for this question. The gas phase decomposition of hydrogen peroxide at 400C H2O2(g)H2O(g)+12O2(g) is second order in H2O2 with a rate constant of 0.650M1s1. If an experiment is performed in which the initial concentration of H2O2 is 0.201M, what is the concentration of H2O2 after 19.0 seconds have passed. 3 item attempts remaining
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


