Question: Use the References to access important values if needed for this question. 2Cr3++3H3AsO4+2H2O3HAsO2+2CrO42+1OH+ In the above reaction, the oxidation state of arsenic changes from to
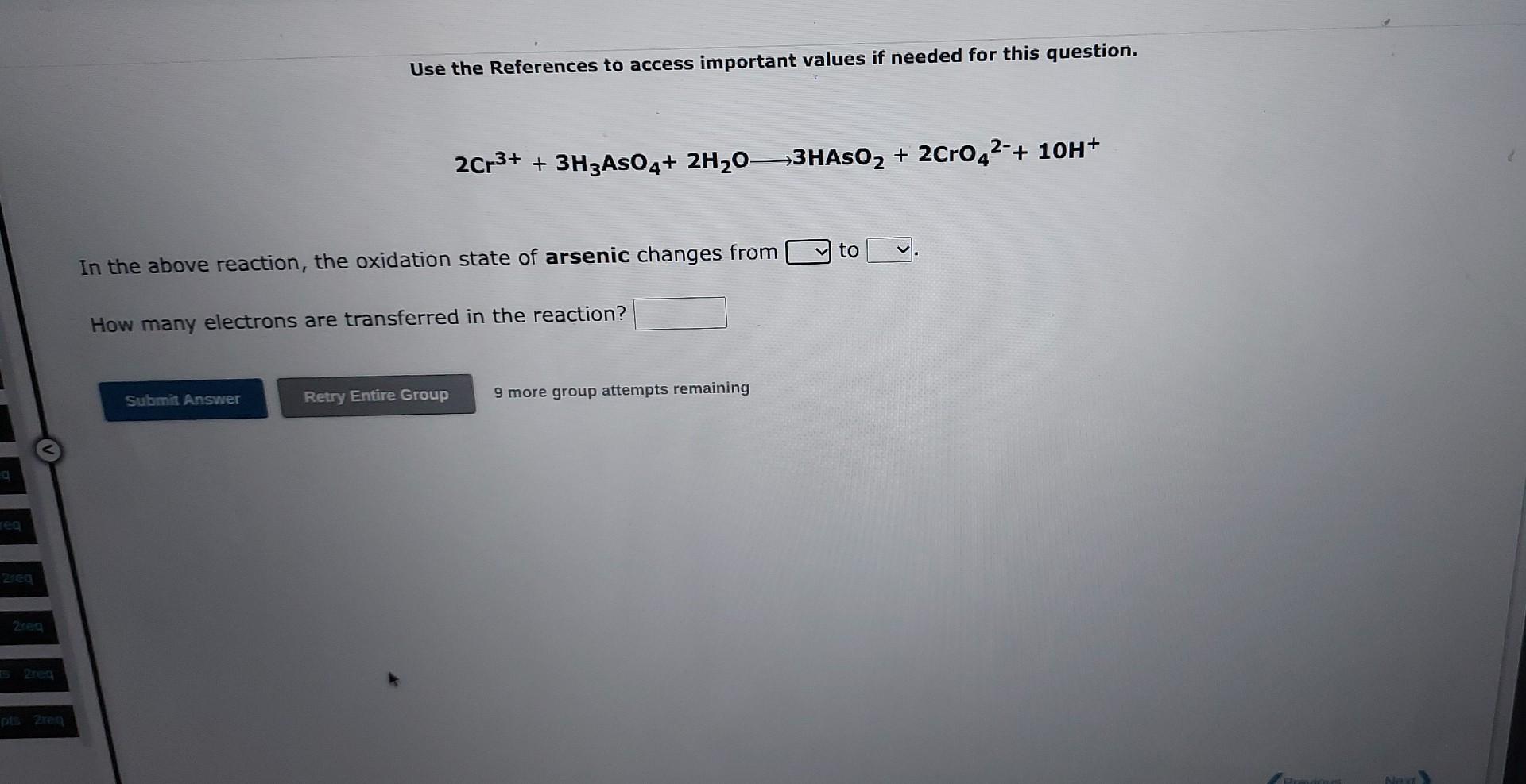
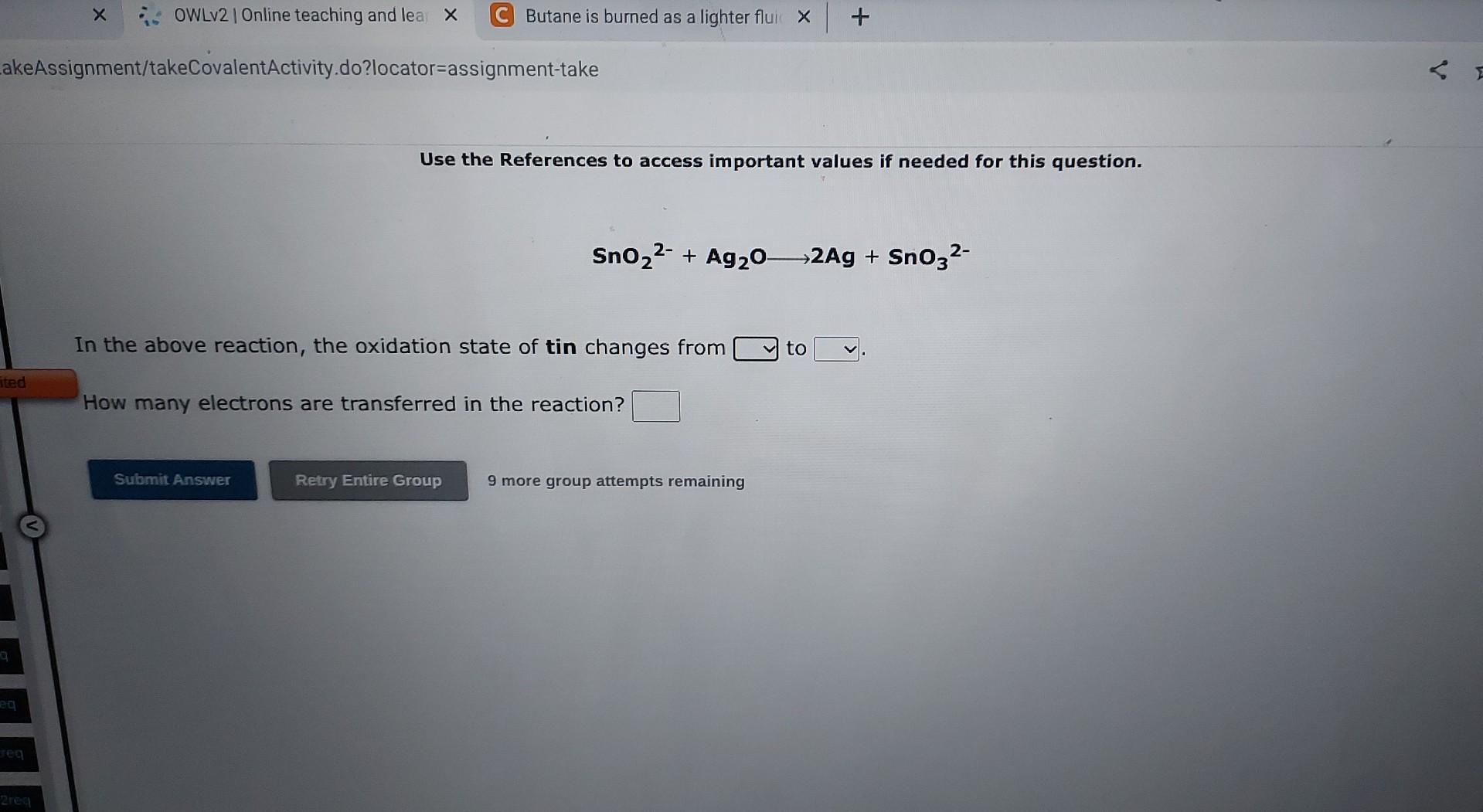
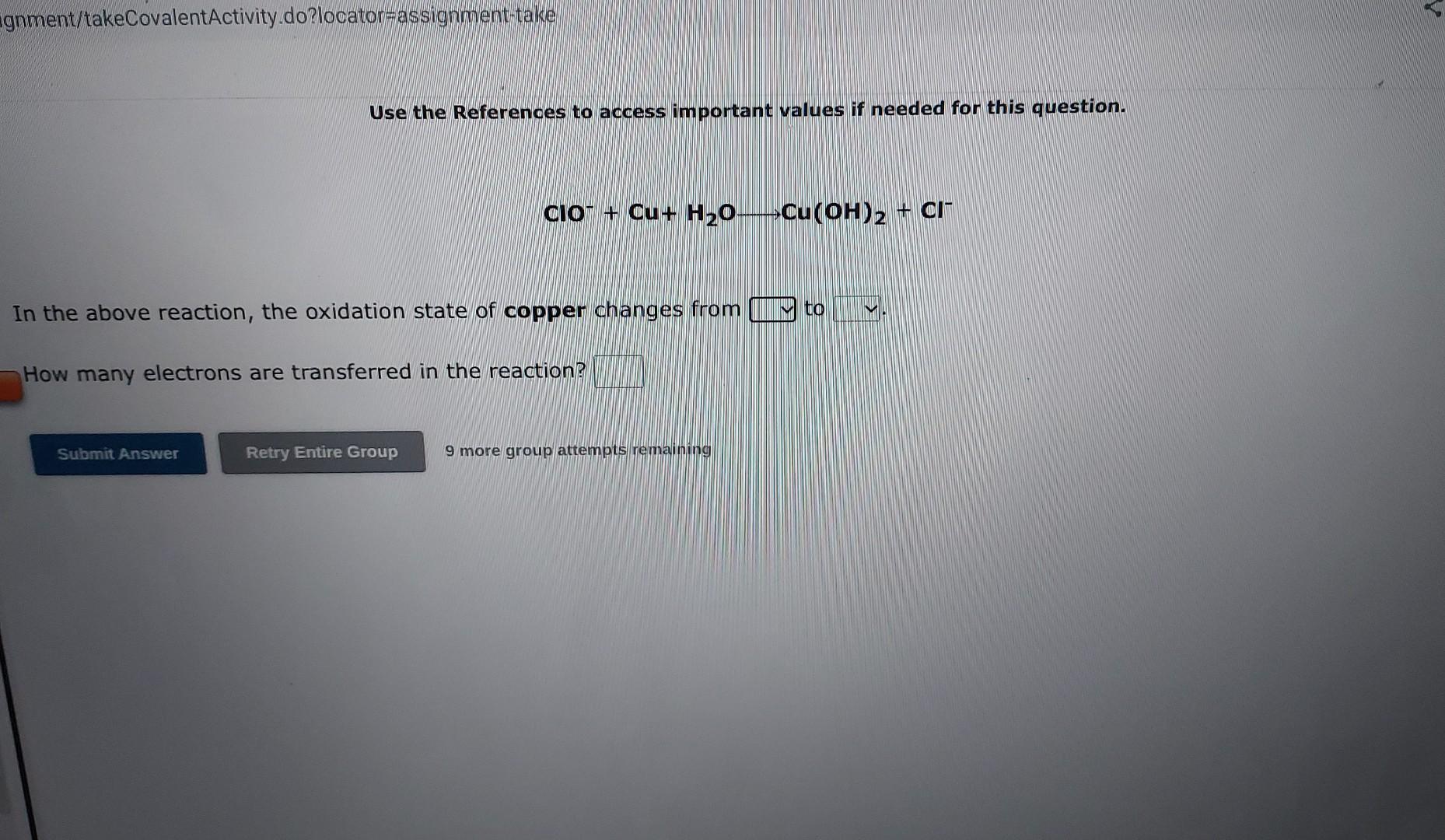
Use the References to access important values if needed for this question. 2Cr3++3H3AsO4+2H2O3HAsO2+2CrO42+1OH+ In the above reaction, the oxidation state of arsenic changes from to How many electrons are transferred in the reaction? 9 more group attempts remaining Use the References to access important values if needed for this question. SnO22+Ag2O2Ag+SnO32 In the above reaction, the oxidation state of tin changes from to How many electrons are transferred in the reaction? 9 more group attempts remaining Use the References to access important values if needed for this question. ClO+Cu+H2OCu(OH)2+Cl
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


