Question: Use the References to access important values if needed for this question. SnO32+Mn(OH)2SnO22+MnO2+H2O In the above reaction, the oxidation state of tin changes from to
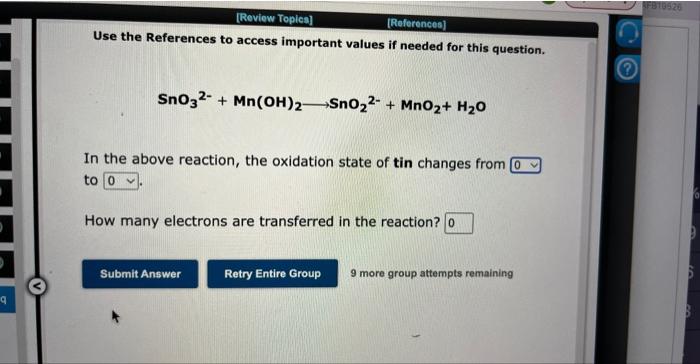
Use the References to access important values if needed for this question. SnO32+Mn(OH)2SnO22+MnO2+H2O In the above reaction, the oxidation state of tin changes from to How many electrons are transferred in the reaction? 9 more group attempts remaining
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


