Question: Use the References to access important values if needed for this question. At 351K and a total equilibrium pressure of 0.957 atm, the fractional dissociation
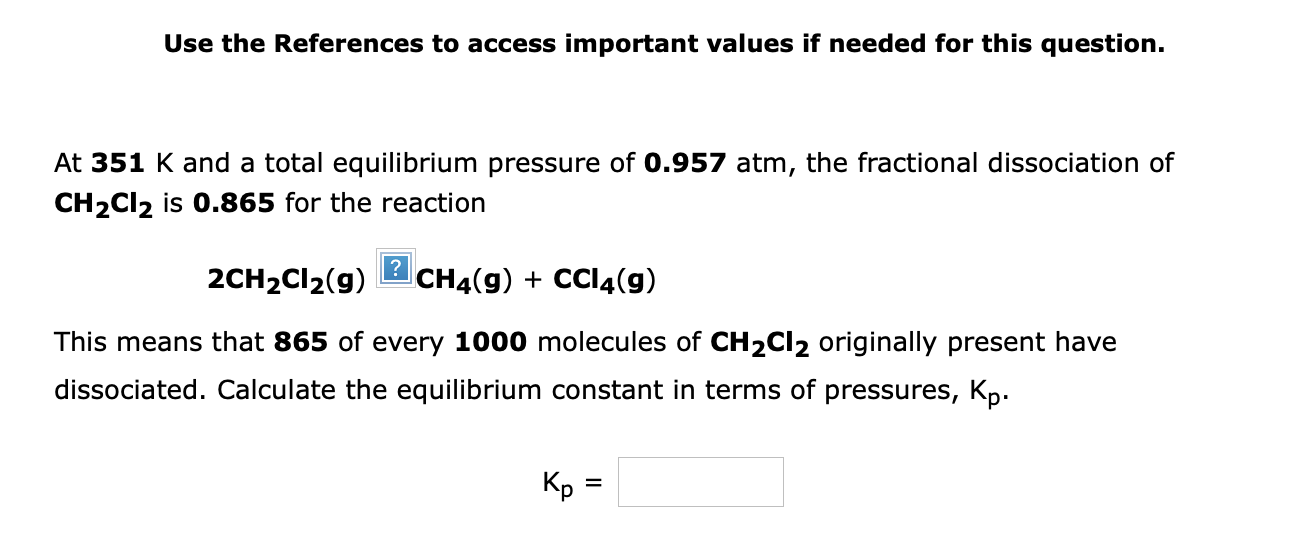
Use the References to access important values if needed for this question. At 351K and a total equilibrium pressure of 0.957 atm, the fractional dissociation of CH2Cl2 is 0.865 for the reaction 2CH2Cl2(g)?CH4(g)+CCl4(g) This means that 865 of every 1000 molecules of CH2Cl2 originally present have dissociated. Calculate the equilibrium constant in terms of pressures, Kp. Kp=
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


