Question: Use the References to access important values if needed for this question. The reaction 2NOBr(g)?2NO(g)+Br2(g) has an equilibrium constant Kp (in terms of pressures) at
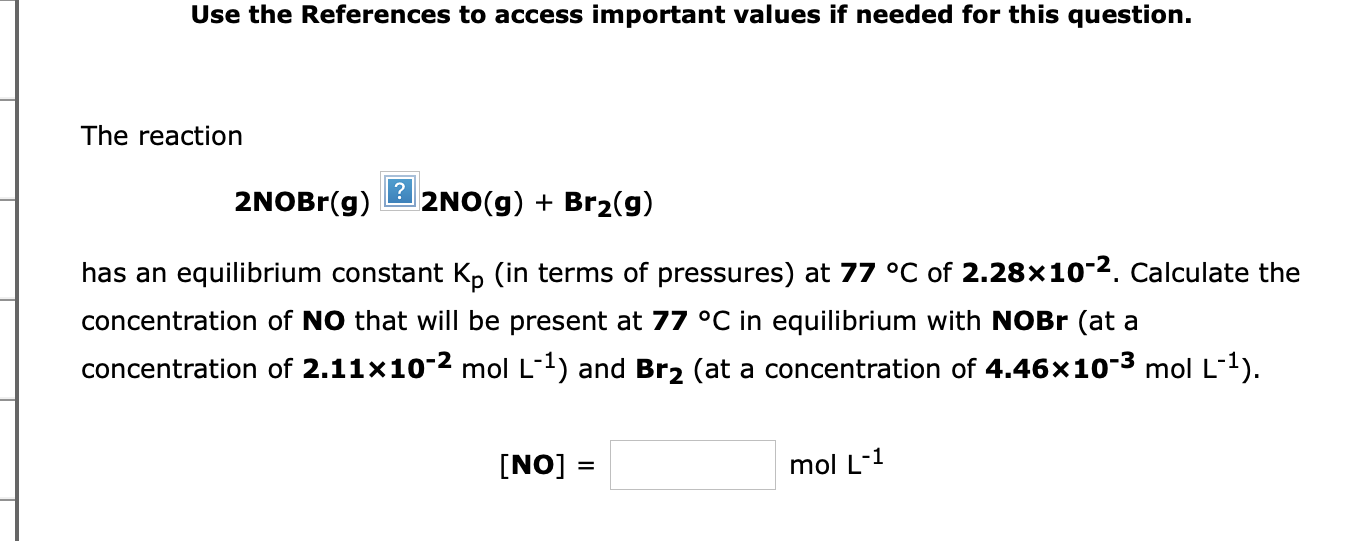
Use the References to access important values if needed for this question. The reaction 2NOBr(g)?2NO(g)+Br2(g) has an equilibrium constant Kp (in terms of pressures) at 77C of 2.28102. Calculate the concentration of NO that will be present at 77C in equilibrium with NOBr (at a concentration of 2.11102molL1 ) and Br2 (at a concentration of 4.46103molL1 )
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


