Question: Use the References to access important values if needed for this question. A chemical reaction is run in which 739 Joules of heat are absorbed
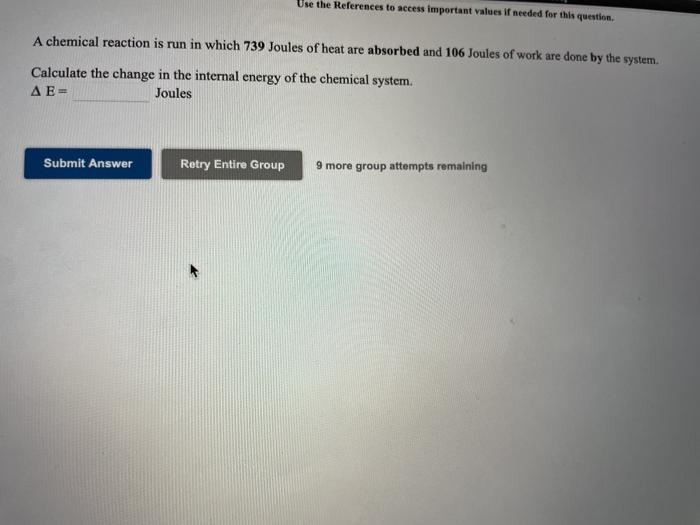
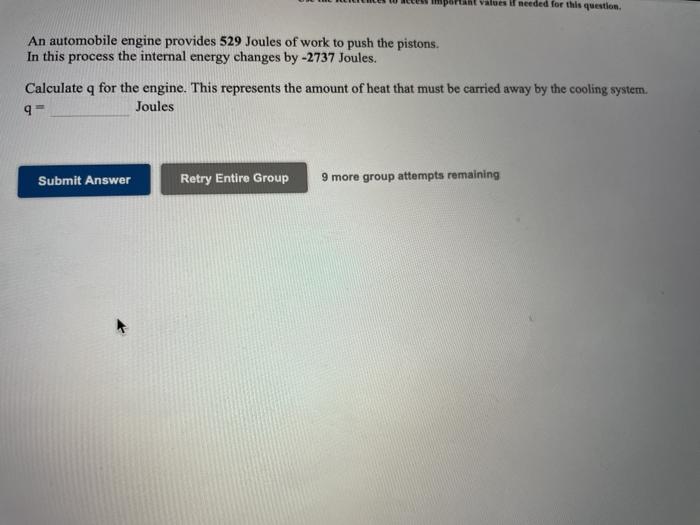
Use the References to access important values if needed for this question. A chemical reaction is run in which 739 Joules of heat are absorbed and 106 Joules of work are done by the system. Calculate the change in the internal energy of the chemical system. Joules Submit Answer Retry Entire Group 9 more group attempts remaining alues i needed for this question. An automobile engine provides 529 Joules of work to push the pistons. In this process the internal energy changes by -2737 Joules. Calculate q for the engine. This represents the amount of heat that must be carried away by the cooling system. 9 Joules Submit Answer Retry Entire Group 9 more group attempts remaining
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


