Question: Using Matlab Recall from Problem 2.7 that the ideal gas law is: PV=nRT and that the Van der Waals modification of the ideal gas law
Using Matlab
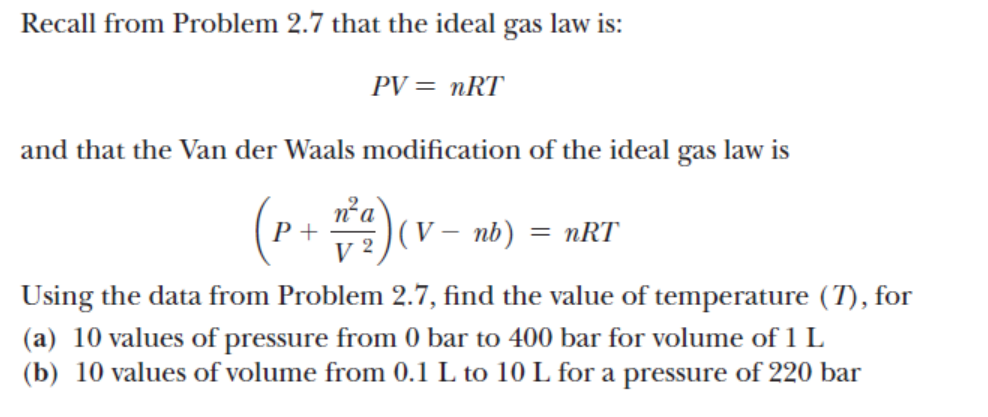
Recall from Problem 2.7 that the ideal gas law is: PV=nRT and that the Van der Waals modification of the ideal gas law is (P+V2n2a)(Vnb)=nRT Using the data from Problem 2.7, find the value of temperature (T), for (a) 10 values of pressure from 0 bar to 400 bar for volume of 1L (b) 10 values of volume from 0.1L to 10L for a pressure of 220bar
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


