Question: How many grams of HO are produced during the combustion of 500 g of glucose C6H12O6 in the presence of 500 g of O?
![]()
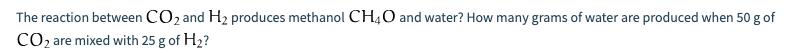

![]()
How many grams of HO are produced during the combustion of 500 g of glucose C6H12O6 in the presence of 500 g of O? The reaction between CO and H produces methanol CH4O and water? How many grams of water are produced when 50 g of CO are mixed with 25 g of H? How many moles of oxygen gas O(g) are needed to produce 108 g of water HO in the presence of excess hydrogen gas O(g) How many grams of hydrogen H are needed to react in the presence of excess nitrogen N to produce 119 g of ammonia NH3?
Step by Step Solution
3.35 Rating (155 Votes )
There are 3 Steps involved in it
ANSWER Hot of The combustion of Glucose in presente is ities CH 06 602 ... View full answer

Get step-by-step solutions from verified subject matter experts


