Question: Watch the ChemTour animation below on acid-base titrations. Then, answer the questions about the following reaction: Ca(OH)2(aq)+2HCl(aq)CaCl2(aq)+H2O(l) An aqueous solution of Ca(OH)2 with a concentration
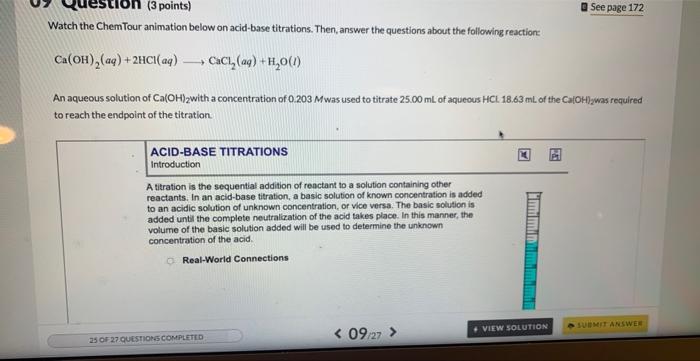
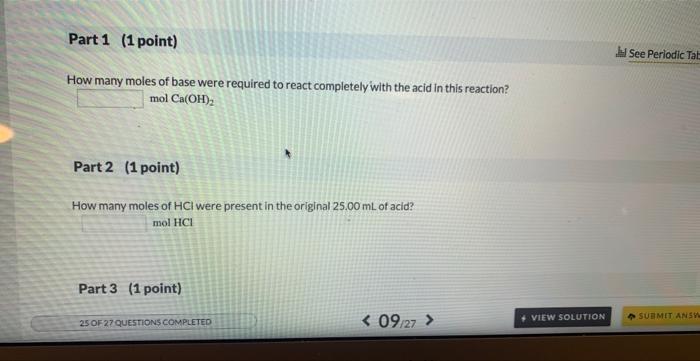
Watch the ChemTour animation below on acid-base titrations. Then, answer the questions about the following reaction: Ca(OH)2(aq)+2HCl(aq)CaCl2(aq)+H2O(l) An aqueous solution of Ca(OH)2 with a concentration of 0.203 M was used to titrate 25.00mL of aqueous HCl.18.63mL of the Ca(OH)2 was required to reach the endpoint of the titration. ACID-BASE TITRATIONS Introduction A titration is the sequential addition of reactant to a solution containing other reactants. In an acid-base titration, a basic solution of known concentration is added to an acidic solution of unknown concentration, of vice versa. The basic solution is added until the complete neutralization of the acid takes place. In this mannec, the volume of the basic solution added will be used to determine the unknown concentration of the acid. Real-World Connections How many moles of base were required to react completely with the acid in this reaction? molCa(OH)2 Part 2 (1 point) How many moles of HCl were present in the original 25.00mL of acid? mol HCl
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


