Question: what else do u need Derivative graph of the pH vs. Volume data represent the equivalence points. Record the pH and volume of NaOH added

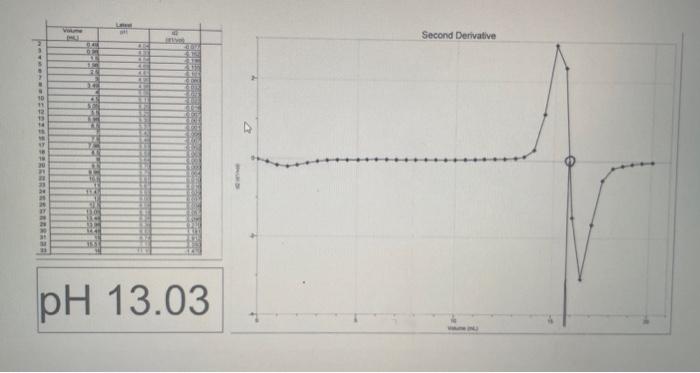
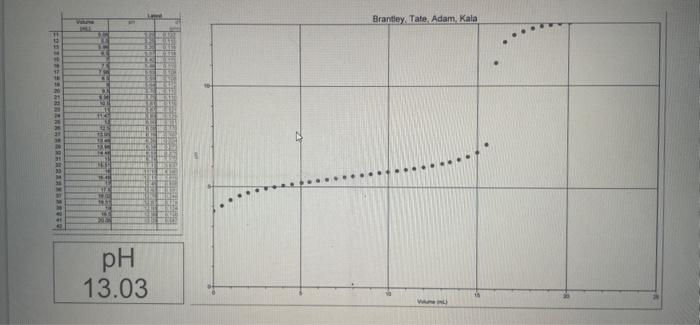
Derivative graph of the pH vs. Volume data represent the equivalence points. Record the pH and volume of NaOH added at the equivalence point. Divide the volume of NaOH required to reach the equivalence point by two to find the half-equivalence point. Using the volume of NaOH at the half-equivalence point, reach the pH at the half-equivalence point from the pH vs. Volume of NaOH graph. Record the pH and volume of NaOH added at both the equivalence point and the half-equivalence point in your notebook. Calculate the pKa and Ka of the weak acid and report these values in your notebook. Use the graphs at the end of procedure for the necessary data to complete the lab calculations. Report the \% difference between your caiculated ph value and the accepted pK2 value of 4.76. Submit your lab report online as either a word doc or pdf fle
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


