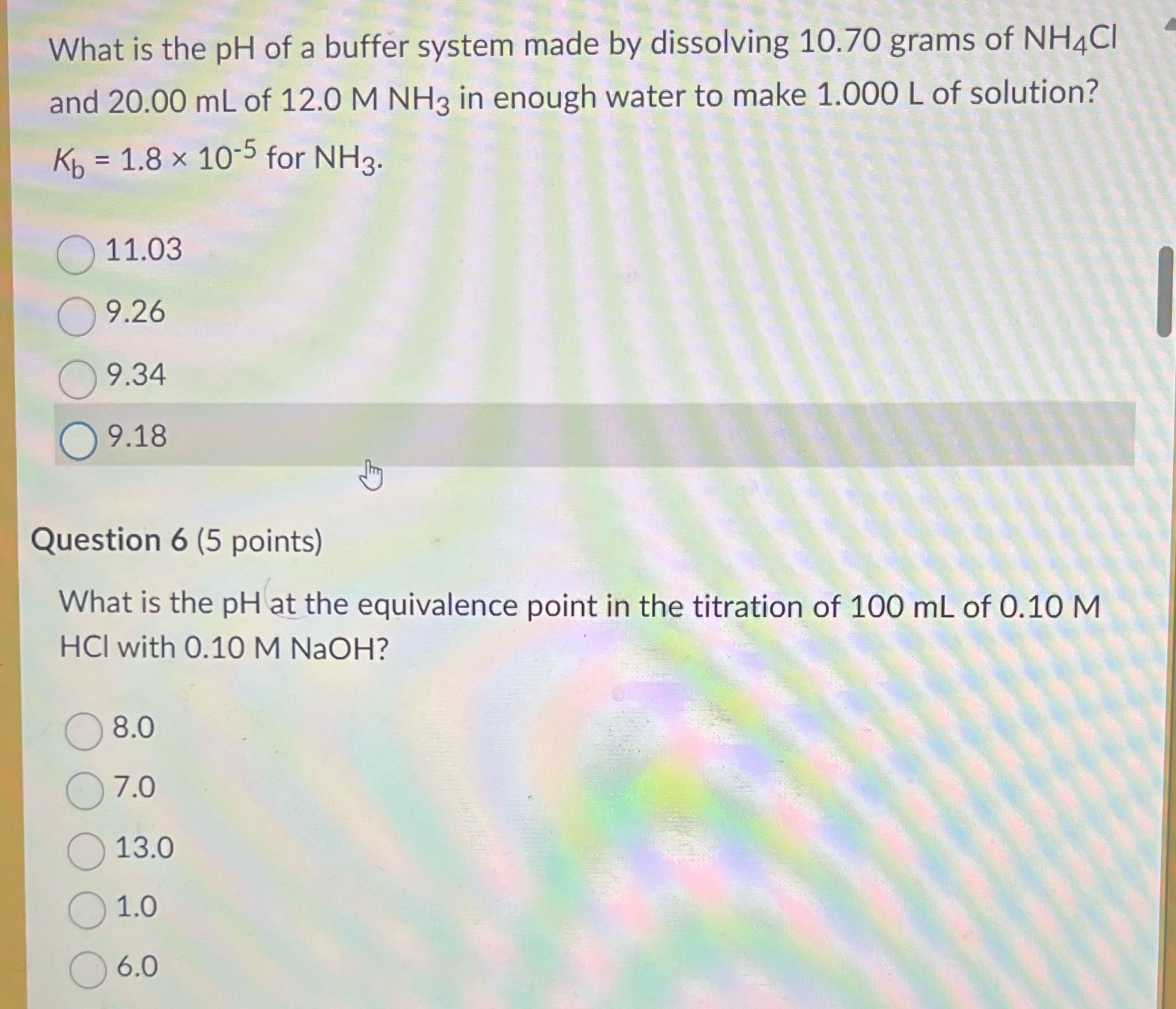
Question: What is the pH of a buffer system made by dissolving 10.70 grams of NH4CI and 20.00 mL of 12.0 M NH3 in enough water
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


