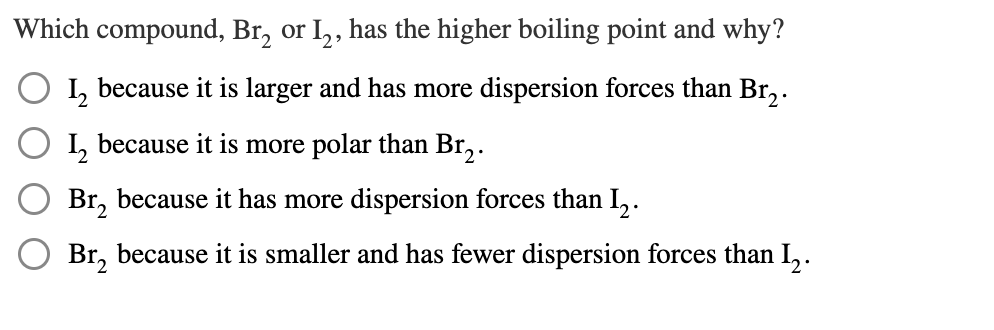
Question: Which compound, Br_(2) or I_(2) , has the higher boiling point and why? I_(2) because it is larger and has more dispersion forces than Br_(2)
Which compound,
Br_(2)or
I_(2), has the higher boiling point and why?\
I_(2)because it is larger and has more dispersion forces than
Br_(2).\
I_(2)because it is more polar than
Br_(2).\
Br_(2)because it has more dispersion forces than
I_(2).\
Br_(2)because it is smaller and has fewer dispersion forces than
I_(2).
Which compound, Br2 or I2, has the higher boiling point and why? I2 because it is larger and has more dispersion forces than Br2. I2 because it is more polar than Br2. Br2 because it has more dispersion forces than I2. Br2 because it is smaller and has fewer dispersion forces than I2
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


