Question: Which of the structures below would be the more stable resonance form, and why? A B, the most electronegative element bears the negative charge A,
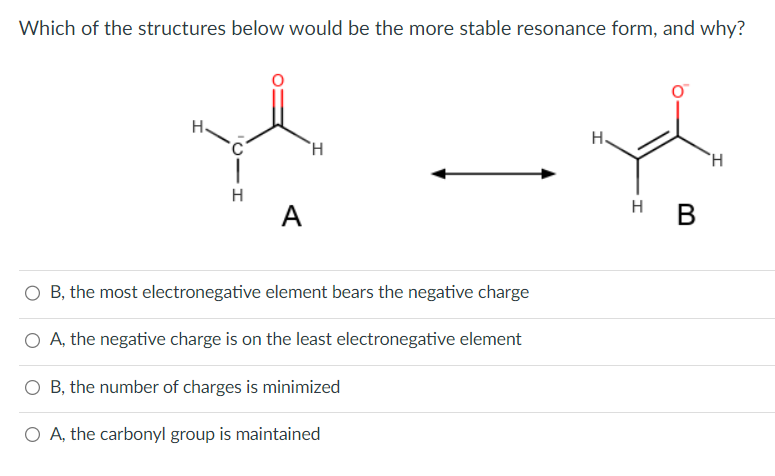
Which of the structures below would be the more stable resonance form, and why? A B, the most electronegative element bears the negative charge A, the negative charge is on the least electronegative element B, the number of charges is minimized A, the carbonyl group is maintained
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


