Question: which one is more stable (In this equation A, B, C and D stand for some unknown chemical formulas.) Here is an energy diagram for
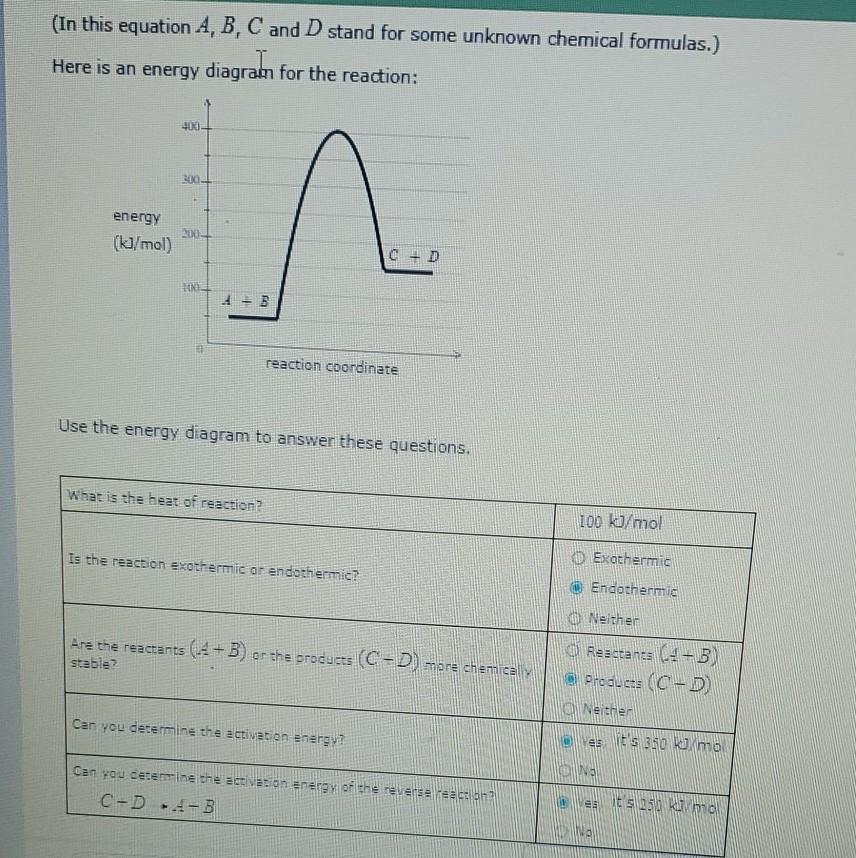
which one is more stable
(In this equation A, B, C and D stand for some unknown chemical formulas.) Here is an energy diagram for the reaction: 40x0 2002 energy (kJ/mol) 200 C D HE 1 B reaction coordinate Use the energy diagram to answer these questions. What is the heat of reaction 100 kJ/mol is the reaction exothermic or endothermicz Exothermic Endothermic Are the reactants (-4-B) or the products (C-D) more chemical stable? Neither Reactan (4-3) Products (C-D Nether as it's 350 kJ/mo Can you determine the activation energy Na Can you determine the action Energy of the reverse action C-D--B ests 250 amor
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


