Question: Which statement is true regarding acids and bases? NaOH is an Arrhenius acid and a Bronsted-Lowry base. KOH is a Bronsted-Lowry base but not an
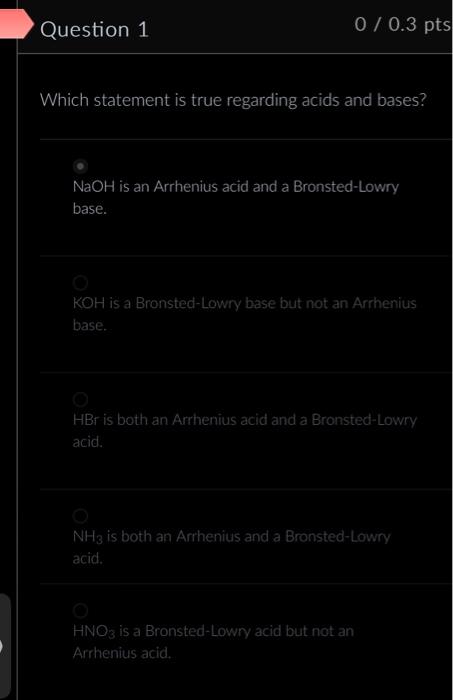
Which statement is true regarding acids and bases? NaOH is an Arrhenius acid and a Bronsted-Lowry base. KOH is a Bronsted-Lowry base but not an Arrhenius base. HBr is both an Arrhenius acid and a Bronsted-Lowry acid. NH3 is both an Arrhenius and a Bronsted-Lowry acid. HNO3 is a Bronsted-Lowry acid but not an Arrhenius acid
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


