Question: A student wants to determine the rate law for the generic reaction below. Using the data below, determine the rate law for the reaction
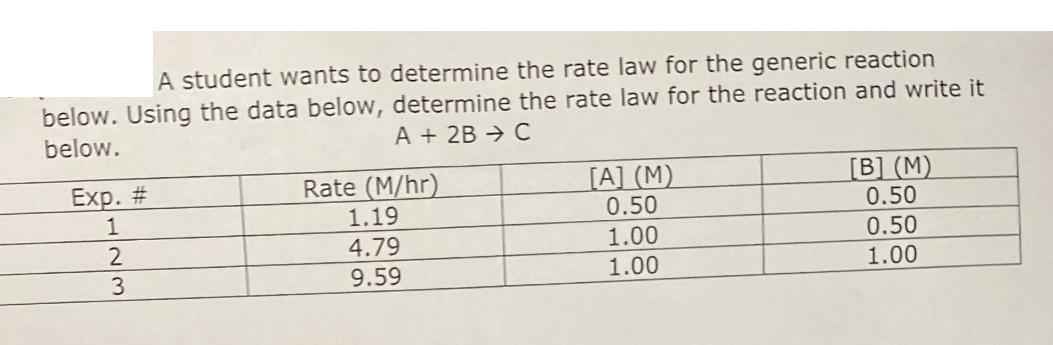
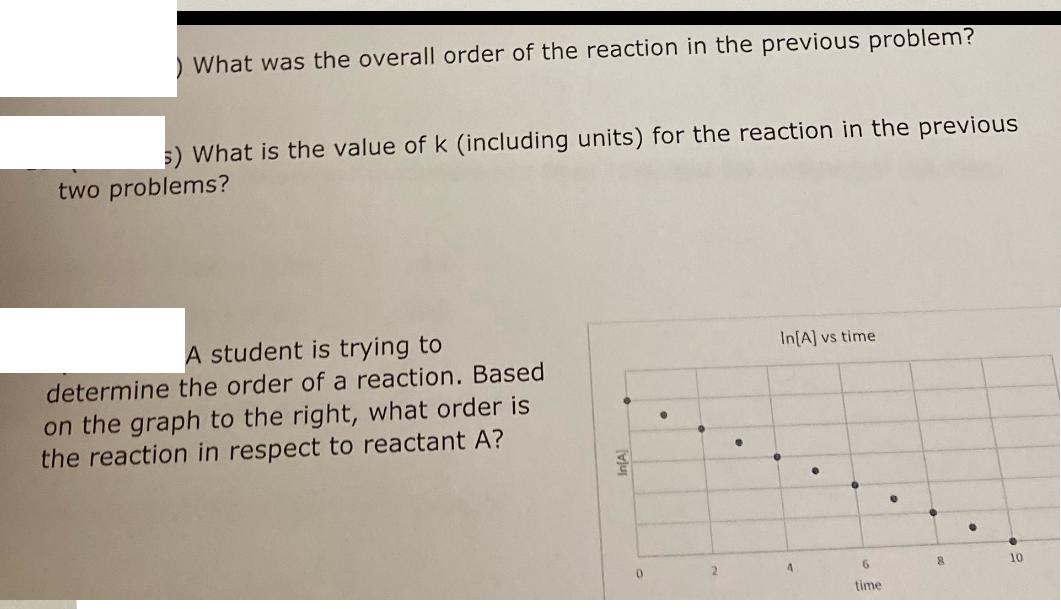
A student wants to determine the rate law for the generic reaction below. Using the data below, determine the rate law for the reaction and write it below. Exp. # 1 2 3 A+ 2B C Rate (M/hr) [A] (M) [B] (M) 1.19 0.50 0.50 4.79 1.00 0.50 9.59 1.00 1.00 What was the overall order of the reaction in the previous problem? 5) What is the value of k (including units) for the reaction in the previous two problems? A student is trying to determine the order of a reaction. Based on the graph to the right, what order is the reaction in respect to reactant A? In[A] vs time 9 D 4 6 8 time 10
Step by Step Solution
3.50 Rating (157 Votes )
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


