Question: Write a balanced chemical equation (smallest integer coefficients possible) for the reaction between an acid and a base that leads to the production of Fe2S3(s).
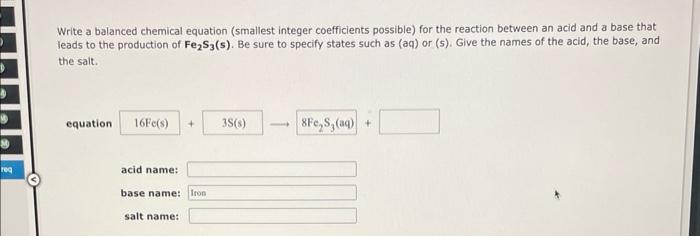
Write a balanced chemical equation (smallest integer coefficients possible) for the reaction between an acid and a base that leads to the production of Fe2S3(s). Be sure to specify states such as (aq) or (s). Give the names of the acid, the base, and the salt. equation acid name: base name
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


