Question: Write a net ionic equation to show that formic acid, HCOOH, behaves as a Brnsted-Lowry acid in water
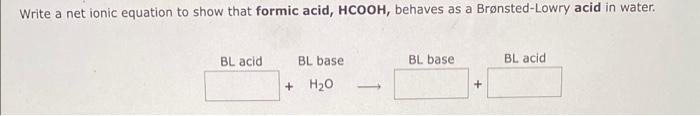
Write a net ionic equation to show that formic acid, HCOOH, behaves as a Brnsted-Lowry acid in water
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


