Question: Write a net ionic equation to show that trimethylamine, (CH3)3N behaves as a Bronsted-Lowry base in water. (For organic molecules enter elements in order they
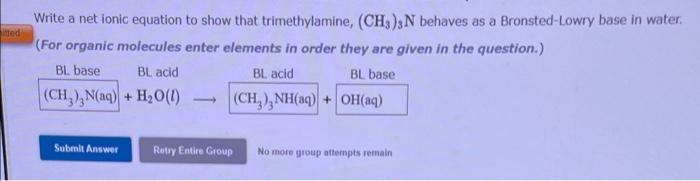

Write a net ionic equation to show that trimethylamine, (CH3)3N behaves as a Bronsted-Lowry base in water. (For organic molecules enter elements in order they are given in the question.) BL base BLacid+H2O(l) BL acid BL base Write a net ionic equation to show that hydroxylamine, NH2OH behaves as a Bronsted-Lowry base in water. (For organic molecules enter elements in order they are given in the question.) BLbacid+H2O(l)BLacidBLbase
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


