Question: Write a net ionic equation to show that quinoline, C9H7N behaves as a Bronsted-Lowry base in water. (For organic molecules enter elements in order they


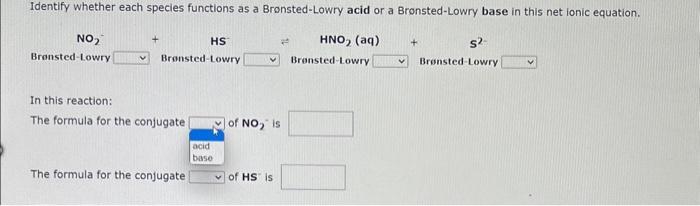
Write a net ionic equation to show that quinoline, C9H7N behaves as a Bronsted-Lowry base in water. (For organic molecules enter elements in order they are given in the question.) Write a net ionic equation to show that formic acid, HCOOH, behaves as a Bronsted-Lowry acid in water. Identify whether each species functions as a Bronsted-Lowry acid or a Bronsted-Lowry base in this net ionic equation. In this reaction: The formula for the conjugate of NO2is The formula for the conjugate of HS15
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


