Question: Write Report Example 4 In the process shown below, the feed stream is composed of 50% (mol) HCl, 48% (mol) C2H4 and 2% (mol) N2

Write Report
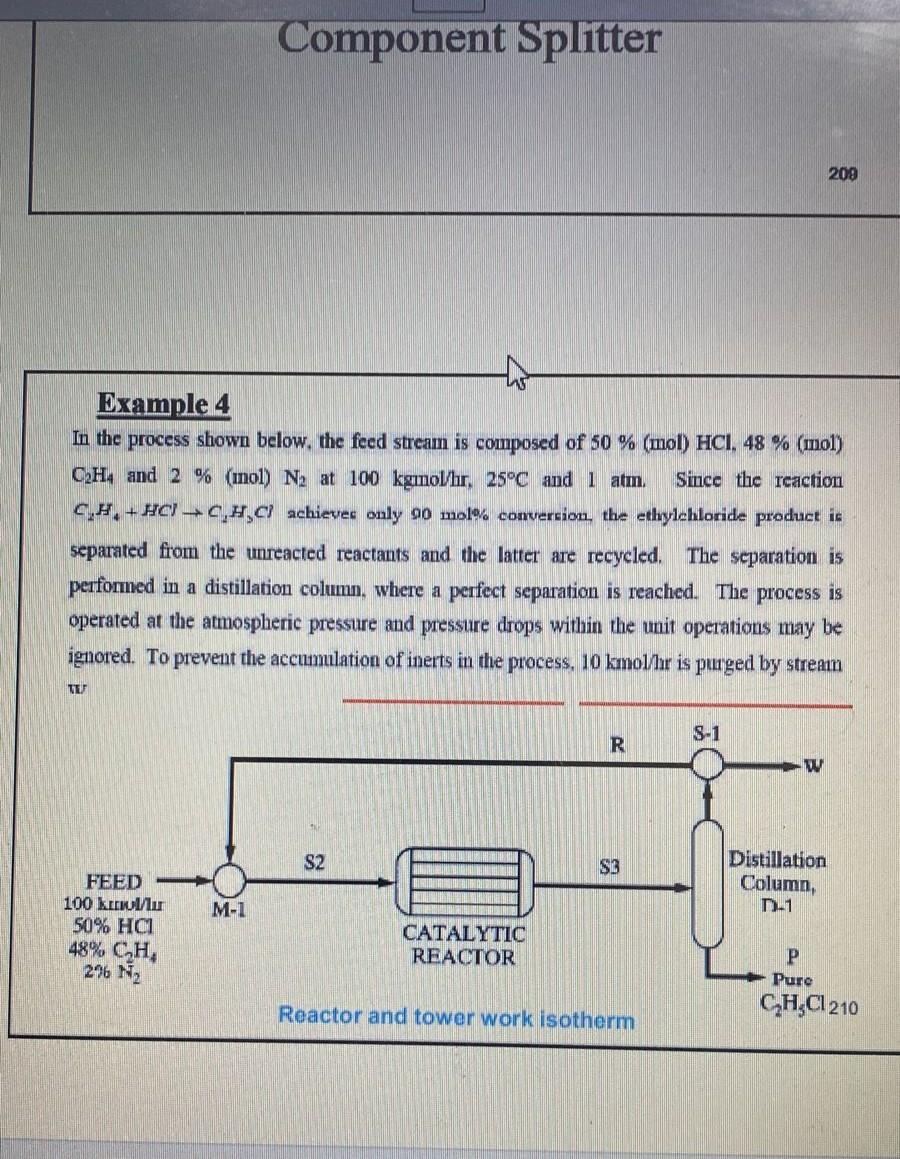
Example 4 In the process shown below, the feed stream is composed of 50% (mol) HCl, 48% (mol) C2H4 and 2% (mol) N2 at 100kgmol/hr,25C and 1atm. Since the reaction C2H4+HClC2H3Cl achievec only 90 mol\% convercion, the ethylehloride product is separated from the unreacted reactants and the latter are recycled. The separation is performed in a distillation column, where a perfect separation is reached. The process is operated at the atmospheric pressure and pressure drops within the unit operations may be ignored. To prevent the accumulation of inerts in the process, 10kmol/7r is purged by stream wV SIMULATION LAB SHEET-6 Example-4 Component Splitter
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


