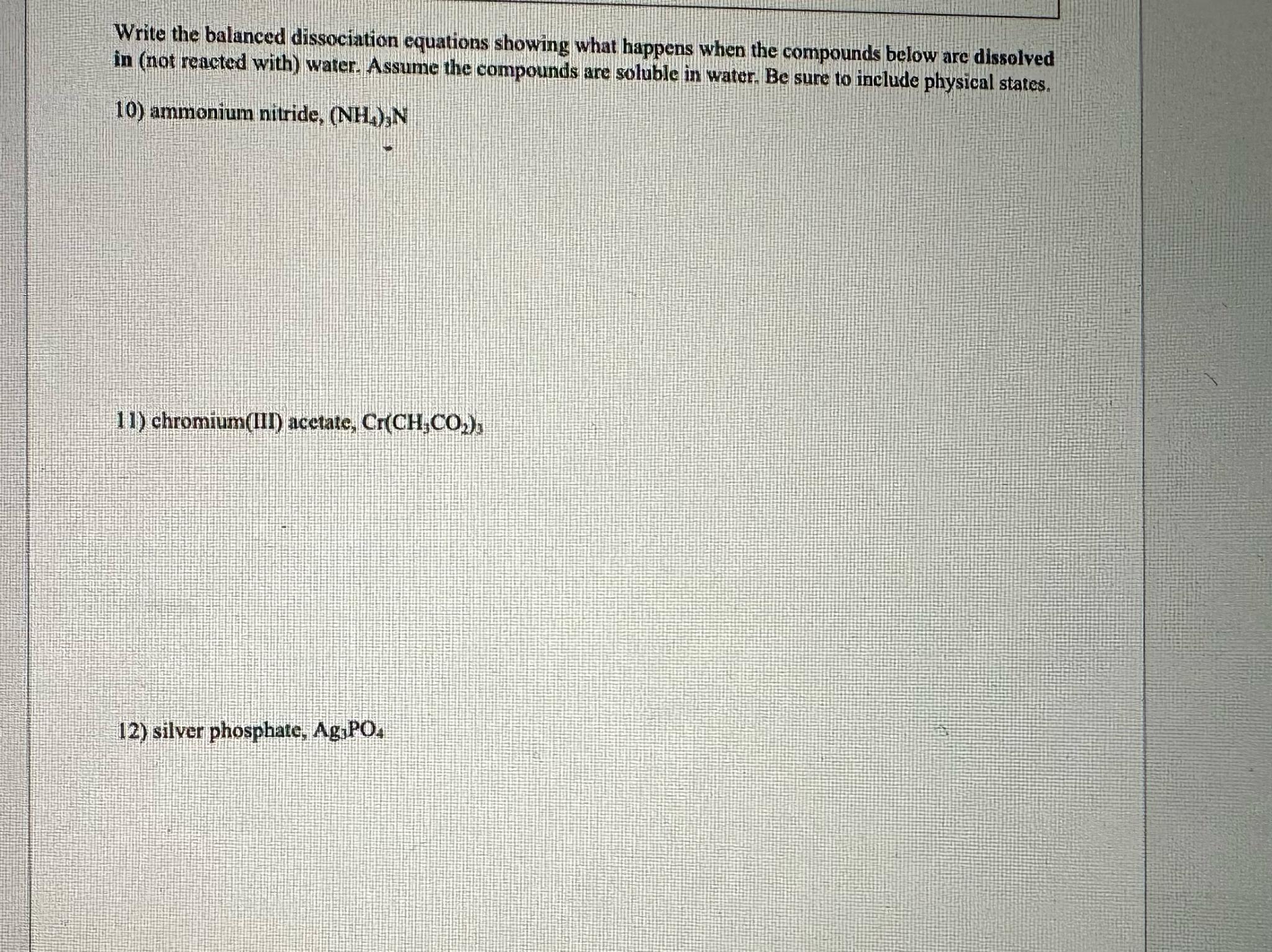
Question: Write the balanced dissociation equations showing what happens when the compounds below are dissolved in ( not reacted with ) water. Assume the compounds are
Write the balanced dissociation equations showing what happens when the compounds below are dissolved in not reacted with water. Assume the compounds are soluble in water. Be sure to include physical states.
ammonium nitride,
chromium III acetate,
silver phosphate,
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


