Question: You make a solution using a solid solute (5g ) in water (total volume 100mL ). The molar mass of the solute is 110g/mol. What
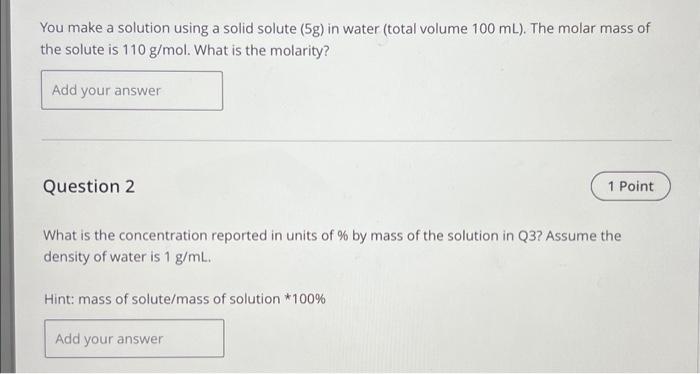
You make a solution using a solid solute (5g ) in water (total volume 100mL ). The molar mass of the solute is 110g/mol. What is the molarity? Question 2 What is the concentration reported in units of % by mass of the solution in Q3? Assume the density of water is 1g/mL. Hint: mass of solute/mass of solution * 100%
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


